This article has been corrected. See "Erratum to "Toll-Like Receptor 4 Signaling is Involved in IgA-Stimulated Mesangial Cell Activation" by Lim BJ, et al. (Yonsei Med J 2011;52:610-5.)" in Volume 55 on page 1747.
Abstract
Purpose
Deposition of polymeric IgA1 in the kidney mesangium is the hallmark of IgA nephropathy, but the molecular mechanisms of IgA-mediated mesangial responses and inflammatory injuries remain poorly understood. We hypothesize that Toll-like receptor 4 (TLR4) is involved in IgA-induced mesangial cell activation.
Materials and Methods
Mouse mesangial cells were stimulated with lipopolysaccharide (LPS) (1 µg/mL), IgA (20 µg/mL), or both, and TLR4 expression was measured by real time RT-PCR and Western blot. Intracellular responses to LPS or IgA were assessed by Western blot for ERK1/2, JNK, p38 MAP kinases (MAPKs), Iκ-Bα degradation and fibronectin secretion. MCP-1 secretion was assessed by ELISA. Small interfering RNA (siRNA) of TLR4 was used to confirm that the effects were caused by TLR4 activity.
Results
LPS- or IgA-treatment upregulated the levels of TLR4 mRNA and protein in cultured MMC at 24 h. LPS and IgA induced rapid phosphorylation of MAPKs, but degradation of Iκ-Bα was observed only in LPS-treated MMC. LPS, but not IgA, induced increased secretion of MCP-1 and fibronectin at 24 h or 48 h. Combined LPS and IgA treatment did not cause additional increases in TLR4 mRNA and protein levels or Iκ-Bα degradation, and MCP-1 and fibronectin secretions were less than with LPS alone. LPS- or IgA-induced TLR4 protein levels and MAPK activation were inhibited by transfection with TLR4 siRNA.
Toll-like receptors (TLRs) are a family of pathogen pattern recognition receptors that activate defense mechanisms in response to exposure to various bacteria and viruses. Research in this field has rapidly expanded since the first discovery of TLR4 in 1997, with 11 currently recognized subtypes in humans and 13 in mice.1,2 TLRs may respond to endogenous substances produced during tissue injury without exogenous stimuli, and they may not always be as protective as in native immunity. Various TLRs are distributed in different cell types either constitutively or in response to various conditions. In the kidney, TLR4 and probably TLR11 help to recognize some strains of uropathogenic bacteria in urothelial cells.3 Other known relationships include TLR2 and TLR4 in ischemia/reperfusion injury of renal tubular epithelial cells, TLR4 in renal allograft rejection, and TLR3, TLR7 and TLR9 in immune complex-mediated glomerulonephritides.4
IgA nephropathy (IgAN) is one of the most common forms of glomerulonephritis worldwide, and is characterized by mesangial expansion, mesangial proliferation, and predominant deposition of IgA in the mesangium. IgA deposited in the mesangium is deemed mainly responsible for functional derangement and histologic alteration. The molecular mechanisms of IgA-mediated mesangial responses and the initiation of inflammatory injury remain poorly understood. There has been circumstantial but supportive evidence for the involvement of TLRs in the glomerular injury of IgAN. First, tonsillar mononuclear cells of IgAN patients produce higher amounts of monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-8 than tonsils from non-IgAN patients when incubated with staphylococcus enterotoxin-B or lipopolysaccharide (LPS).5 Upregulation of TLR4 expression in circulating mononuclear cells of IgAN patients is associated with proteinuria and heavy microscopic hematuria of IgAN patients.6 Second, glomerular mesangial cells express TLR1-4 and TLR6,7 and may promote CXC cytokines upon TLR4 activation.8 Third, levels of some endogenous TLR4 ligands such as fibronectin and fibrinogen increase in the blood or are frequently co-deposited in the glomerular mesangium of IgAN.9 Fourth, TLR4 and TLR9 expression increases in renal extracts of post-transplant IgAN patients.10 TLR9 polymorphisms are associated with disease severity in patients with IgAN.11 Nonetheless, there is no direct evidence for TLR involvement in mesangial cell activation in IgAN. In this study, we explored TLR4 involvement in IgA-stimulated mesangial cell activation and evaluated whether LPS caused additive or synergistic effects in IgA-induced mesangial cell activation.
Mouse mesangial cells (MMC) from an SV40 transgenic mouse (MES-13) were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Gaithersburg, MD, USA) containing 5% fetal bovine serum (Invitrogen). MMC were maintained and incubated at 37℃ in humidified 5% CO2 in air. Nearly confluent MMC were incubated in serum-free media for 16 h to arrest and synchronize cell growth. MMC were stimulated with LPS (Sigma, Poole, UK), IgA (Sigma, I2636, human secretory IgA, 400 kDa), or both for up to 48 h. Doses of 1 µg/mL for LPS and 20 µg/mL for IgA were chosen for the experiment after serial dose testing from 10 ng/mL to 10 µg/mL for LPS and 10 µg/mL to 200 µg/mL for IgA. Cell viability was not significantly altered in the doses chosen for experiments, which was confirmed by the trypan blue exclusion test. Cells were pre-treated with inhibitors of ERK, JNK, and p38 MAP kinases (MAPKs) (Calbiochem, San Diego, CA, USA; 10 µM each) for 60 min prior to LPS or IgA stimulation.
Total RNA was extracted from cultured MMC by Trizol (Invitrogen) extraction, and 4 µg of isolated RNA were reverse-transcribed into cDNA using a high-capacity cDNA synthesis kit (iNtRON biotechnology, Seoul, Korea). Real-time PCR was performed with the ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR Master Mix (Applied Biosystems) in accordance with the manufacturer's instructions. PCR cycling conditions included pre-denaturing at 95℃ for 15 s, then 35 cycles of denaturation at 95℃ for 5 s, and combined annealing/extension at 61℃ for 30 s. Semilog amplification curves were evaluated using the comparative quantification method (2-ΔΔCt), and GAPDH was used for normalization of all reported gene expression levels. The primer sequences used in the present study were as follows: TLR4: forward CATTCAAGACCAAGCCTTTCAG and reverse CCAGGTTTTGAAGGCAAGTTTT. GAPDH: forward TGCACCACCAACTGCTTAG and reverse GGATGC AGGGATGATGTTC.
Harvested MMC were lysed in sodium dodecyl sulfate (SDS) sample buffer [2% SDS, 10 mM Tris-HCl, pH 6.8, 10% (vol/vol) glycerol]. The samples were treated with Laemmli sample buffer, heated at 100℃ for 5 min, and electrophoresed in a 10% acrylamide denaturing SDS-polyacrylamide gel. Proteins were then transferred to a Hybond-ECL membrane using a Hoeffer semidry blotting apparatus (Hoeffer Instruments, San Francisco, CA, USA), and the membrane was incubated in blocking buffer (1×TBS, 0.1% Tween-20, and 5% nonfat milk) for 1 h at room temperature, followed by an overnight incubation at 4℃ in a 1:1000 dilution of antibodies to p-ERK, p-JNK, p-p38, Iκ-Bα (Cell Signaling Technology, Danvers, MA, USA), fibronectin (Dako, Carpinteria, CA, USA), or β-actin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The membrane was then washed once for 15 min and twice for 5 min in 1×TBS with 0.1% Tween-20. Next, the membrane was incubated with horseradish peroxidase-linked goat anti-rabbit IgG (Santa Cruz Biotechnology). The washes were repeated, and the membrane was developed with a chemiluminescent agent (ECL; Amersham Life Science Inc., Arlington Heights, IL, USA). Proteins were quantified using a densitometer.
The levels of MCP-1 secreted into the culture media under different experimental conditions were determined using a commercial MCP-1 ELISA kit (R&D Systems, Abingdon, UK), sensitive to 2 pg/mL, according to the manufacturer's protocol.
Pre-designed mouse TLR4 siRNA was purchased from Ambion (Austin, TX, USA). TLR4 siRNA 25nM was transfected using the lipofectamine method (Invitrogen) for 6 hours. Media were then replaced with fresh serum-free media containing 10 µg/mL LPS or 20 µg/mL IgA, and the cells were incubated for up to 24 h.
LPS increased TLR4 mRNA levels 3.5-fold at 12 h and 3.2-fold at 24 h in MMC. TLR4 protein levels increased 2-fold by 24 h and slightly more by 48 h. IgA upregulated the level of TLR4 mRNA by 3-fold at 24 h, and the level of proteins 3.6- and 1.5b-fold at 24 h and 48 h, respectively. When MMC were stimulated with LPS and IgA together, there was no additive increase in TLR4 mRNA and protein expression at 24 h. At 12 h, the effect of LPS on TLR4 mRNA was even abrogated by IgA cotreatment to the level of IgA alone (Figs. 1 and 2).
LPS induced phosphorylation of ERK1/2, JNK, and p38, and degradation of Iκ-Bα at 5 min, but pre-treatment with inhibitors of ERK1/2, JNK, and p38 inhibited these reactions. IgA and combined IgA and LPS treatment also induced rapid phosphorylation of ERK1/2, JNK, and p38 MAPKs at 5 min (Fig. 3). Iκ-Bα degradation was observed at 60 min in LPS-treated MMC, but not in IgA- and combined LPS and IgA-treated MMC (Fig. 4).
MCP-1 secretion in the culture media increased 1.2-fold at 48 h in LPS-treated MMC. IgA treatment caused an increase MCP-1 secretion at 6 h, but this decreased by 48 h. MCP-1 secretion at 48 h was significantly lower after combined LPS and IgA treatment than after LPS or IgA treatment alone (Fig. 5). Fibronectin secretion increased 1.7- and 1.6-fold at 24 h and 48 h, respectively, in LPS-treated MMC. IgA- and Combined IgA and LPS-treated MMC displayed less fibronectin secretion than MMC treated with LPS alone (Fig. 6).
LPS- or IgA-induced mesangial cellular TLR4 expression and MAPK activation were all inhibited by transfection with TLR4 siRNA (Fig. 7).
Although progress has been made in understanding the structural abnormalities of IgA molecules in IgAN,12 the molecular mechanisms behind mesangial activation by IgA remain poorly understood. Since infections can trigger symptoms and raise serum IgA levels in IgAN patients, we hypothesized that TLR4 may be involved in IgA-induced mesangial activation. In this study, cultured MMC showed elevated TLR4 mRNA and protein levels when stimulated with IgA. TLR4 protein production was observed at 48 h, while TLR4 mRNA was elevated from 24 h, probably due to transcriptional effects.
TLR4 is expressed under basal culture conditions in MMC and distal tubular cells.7 Upregulation of TLR4 expression by angiotensin II has been reported in mouse mesangial cells.13 Our results demonstrate that IgA is another agonist of TLR4 expression in mesangial cells, although the mechanism is still unclear. Cytokines produced by activated mesangial cells in response to IgA binding may promote TLR4 expression in mesangial cells. Patole, et al.7 reported, however, that interferon (IFN)-γ and tumor necrosis factor-α upregulate levels of TLR2, TLR3, and TLR6 mRNA, but not of TLR4 mRNA, in mesangial cells. Alternatively, IgA binding may upregulate mesangial TLR4 expression by increasing endogeneous ligands for TLR4. We assessed fibronectin production, but it did not increase following IgA treatment. TLR ligation did not lead clearly to either protection or aggravation. Induction of heat shock protein (HSP) 70 is protective against oxidative injury in mesangial cells,14 whereas increased tubular expression of HSP70 is an indicator of cyclosporine nephrotoxicity.15 Since there are other endogenous ligands and agonists for TLR4 that were not tested in this study, this possibility is still open.
In the TLR4 signaling pathway, MAPK and NFκB activation follows TLR4 activation. We observed MAPK activation in IgA-stimulated MMC, but Iκ-Bα degradation did not occur. The interaction of IgA with Fcα receptors of human mesangial cells can activate NFκB and induce expression and synthesis of MCP-1, IL-8, and IFN-inducible protein 10.16 Expression of MCP-1 and osteopontin can be induced by LPS via the TLR4 signaling pathway in mesangial cells of lupus-prone mice.17 The fact that Iκ-Bα degradation and MCP-1 production were only observed after LPS treatment makes it likely that MCP-1 production occurred via the TLR4-MAPK-NF-κB pathway. Neither NF-κB activation nor persistent MCP-1 production by MMC stimulated by IgA indicates that IgA per se does not have strong proinflammatory activity in this situation.
Initially, we expected additive or synergistic effects of LPS treatment in IgA-stimulated mesangial cells. We found, however, that IgA treatment did not change the effect of LPS treatment, and sometimes even abolished it. The reasons behind the differing effects of the combined treatment are not clear. The possibility that LPS and IgA compete for the same receptor is unlikely, since IgA is known to bind specifically to the mesangial transferrin receptor and LPS recognizes TLR4. Exposure to both IgA and LPS may switch on protective mechanisms in mesangial cells. Human serum IgA has both pro-inflammatory and anti-inflammatory properties in LPS-stimulated monocytes and peripheral blood mononuclear cells.18,19 Shimosawa, et al.20 reported that mesangial TLR4 expression decreased after LPS administration in a high IgA strain of ddY mice, while mesangial proliferation, macrophage infiltration, and MCP-1 mRNA expression increased. The modified mesangial responses to LPS and IgA observed in our study might contribute to the feedback mechanisms maintaining a balance between pro-inflammatory and anti-inflammatory activities.
Our study design has some drawbacks in its attempt to simulate human IgAN. We used unaltered IgA instead of macromolecular IgA, which is known as the major pathogenic molecule in IgA nephropathy in vivo. Our data showed, however, that MMC could respond to unaltered IgA molecules as well. It is unclear whether we would have observed higher responses to aggregated or chemically modified IgA. In vivo, mesangial injury may be governed or potentiated by infiltrating intraglomerular inflammatory cells rather than IgA. An association between urinary dysfunction and TLR4 upregulation in circulating mononuclear cells supports the idea that inflammatory cells play a role in IgAN patients.6 In IgAN, intraglomerular mononuclear cells are associated with proteinuria.21 Intraglomerular inflammatory cells could express TLR7 and TLR9, and TLR9 stimulation with CpG DNA causes Th1 polarization and disease exacerbation in ddY mice.11
In conclusion, the activation of MAPKs and secretion of MCP-1 is mediated, at least in part, by TLR4 in IgA-treated mesangial cells. TLR4 seems to be involved in mesangial cell injury by the induction of pro-inflammatory cytokines in IgA nephropathy. More studies are needed to understand the intraglomerular cross-talk between mesangial and inflammatory cells.
Figures and Tables
Fig. 1
TLR4 mRNA level was upregulated by LPS at 12 hours and 24 hours, and by IgA at 24 hours. Combined LPS and IgA stimulation did not increase the TLR4 mRNA level. *p<0.05 vs. 24 hours control, †p<0.05 vs. 12 hours control, ‡p<0.05 vs. 12 hours LPS. TLR4, Toll-like receptor 4; mRNA, messenger ribonucleic acid; LPS, lipopolysaccharide.

Fig. 2
TLR4 protein expression was increased by LPS or IgA stimulation at 24 hours and 48 hours. However, combined stimulation of LPS and IgA did not show an additive effect. *p<0.05 vs. 48 hours control, †p<0.05 vs. 24 hours control. TLR4, Toll-like receptor 4; mRNA, messenger ribonucleic acid; LPS, lipopolysaccharide.

Fig. 3
LPS, IgA, and combined LPS and IgA treatment induced rapid phosphorylation of ERK1/2, JNK, and p38 MAPKs at 5 minutes. LPS, lipopolysaccharide; MAPKS, MAP kinases.
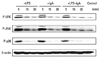
Fig. 4
Iκ-Bα degradation was observed at 60 minutes in LPS-treated MMC, but not in IgA- and combined LPS and IgA-treated MMC. *p<0.05 vs. control. LPS, lipopolysaccharide; MMC, mouse mesangial cells.

Fig. 5
MCP-1 secretion in the culture media increased at 48 hours in LPS-treated MMC, but was significantly lower after treatment with combined LPS and IgA compared to LPS alone. *p<0.05 vs. 48 hours control, †p<0.05 vs. 48 hours LPS, ‡p<0.05 vs. 48 hours IgA. LPS, lipopolysaccharide; MMC, mouse mesangial cells.

References
1. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006. 124:783–801.

2. Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005. 17:338–344.

3. Anders HJ, Schlöndorff D. Toll-like receptors: emerging concepts in kidney disease. Curr Opin Nephrol Hypertens. 2007. 16:177–183.

4. El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol. 2006. 2:568–581.

5. Kuki K, Gotoh H, Hayashi M, Hujihara K, Tamura S, Yamanaka N. Immunity of tonsil and IgA nephropathy--relationship between IgA nephropathy and tonsillitis. Acta Otolaryngol Suppl. 2004. 124:6–9.

6. Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, Loiacono E, et al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol. 2010. 159:73–81.

7. Patole PS, Pawar RD, Lech M, Zecher D, Schmidt H, Segerer S, et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol Dial Transplant. 2006. 21:3062–3073.

8. Brown HJ, Lock HR, Wolfs TG, Buurman WA, Sacks SH, Robson MG. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J Am Soc Nephrol. 2007. 18:1732–1739.

9. Mattii L, Segnani C, Cupisti A, D'Alessandro D, Moscato S, Meola M, et al. Kidney expression of RhoA, TGF-beta1, and fibronectin in human IgA nephropathy. Nephron Exp Nephrol. 2005. 101:e16–e23.
10. Kwon J, Park J, Lee D, Kim YS, Jeong HJ. Toll-like receptor expression in patients with renal allograft dysfunction. Transplant Proc. 2008. 40:3479–3480.

11. Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008. 19:2384–2395.

12. Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995. 100:470–474.

13. Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol. 2006. 17:1585–1593.

14. Chen HC, Guh JY, Tsai JH, Lai YH. Induction of heat shock protein 70 protects mesangial cells against oxidative injury. Kidney Int. 1999. 56:1270–1273.

15. Lim BJ, Hong SW, Jeong HJ. Renal tubular expression of Toll-like receptor 4 in cyclosporine nephrotoxicity. APMIS. 2009. 117:583–591.

16. Duque N, Gómez-Guerrero C, Egido J. Interaction of IgA with Fc alpha receptors of human mesangial cells activates transcription factor nuclear factor-kappa B and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997. 159:3474–3482.
17. Ka SM, Cheng CW, Shui HA, Wu WM, Chang DM, Lin YC, et al. Mesangial cells of lupus-prone mice are sensitive to chemokine production. Arthritis Res Ther. 2007. 9:R67.

18. Wolf HM, Hauber I, Gulle H, Samstag A, Fischer MB, Ahmad RU, et al. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin Exp Immunol. 1996. 105:537–543.

19. Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005. 140:478–490.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download