Abstract
Purpose
To assess the clinical manifestations and multidetector-row computed tomography (MDCT) findings of afferent loop syndrome (ALS) and to determine the role of MDCT on treatment decisions.
Materials and Methods
From January 2004 to December 2008, 1,100 patients had undergone gastroenterostomy reconstruction in our institution. Of these, 22 (2%) patients were diagnosed as ALS after surgery that included Roux-en-Y gastroenterotomy (n=9), Billroth-II gastrojejunostomy (n=7), and Whipple's operation (n=6). Clinical manifestations and MDCT features of these patients were recorded and statistically analyzed. The presumed etiologies of obstruction shown on the MDCT were correlated with clinical information and confirmed by surgery or endoscopic biopsy.
Results
The most common clinical symptom was acute abdominal pain, presenting in 18 patients (82%). We found that a fluid-filled C-shaped afferent loop in combination with valvulae conniventes projecting into the lumen was the most common MDCT features of ALS. Malignant causes of ALS, such as local recurrence and carcinomatosis, are the most common etiologies of obstruction. These etiologies and associated complications can be predicted 100% by MDCT.
Afferent loop syndrome (ALS) is a rare complication of Billroth-II gastrojejunostomy (B-II), Roux-en-Y gastroenterotomy (R-Y), and Whipple's operation. Most cases of ALS are caused by obstructions from adhesion, kinking at the anastomosis, internal hernia, stomal stenosis, malignancy, or inflammation surrounding the anastomosis.1,2 Clinically, ALS is often difficult to diagnose because its presentation may be vague and nonspecific. Delayed diagnosis may result in life-threatening events such as bowel ischemia or perforation. Although conventional barium studies can be helpful to the diagnosis of ALS by allowing visualization of non-filling afferent loops,1 non-obstructed afferent loop are not filled in 20% of cases, and thus the underlying cause of bowel obstruction cannot be thoroughly investigated via barium study.
Multidetector-row computed tomography (MDCT) is now in widespread use, and is believed to function better than conventional CT in diagnosing bowel diseases. It acquires several thin slices during a single rotation of the X-ray tube, thus improving temporal and spatial resolution. This allows for high-quality multiplanar reformations.3 Diagnosis of ALS using coronal reformation makes sense intuitively because it displays more bowel loops in a single image. This should theoretically improve tracing of the bowel loops, which is necessary when evaluating ALS. The display is similar to the plane learned by physicians in their anatomy and surgical training. Although there are many advantages to coronal reformation, to our knowledge the importance of this image display in assessing ALS has never been emphasized before.
In this study, we suggest that MDCT findings of ALS are important for clinical management decisions and can affect treatment policy. Hence, we have conducted a retrospective study to evaluate the clinical manifestations and MDCT findings of ALS and to suggest the underlying causes of ALS by MDCT images.
This study was approved by our Institutional Review Board for Human Investigation, and informed consent was waived due to the retrospective nature of the study. We performed a retrospective computerized search of the medical records at our tertiary referral medical center from January 2004 to December 2008 that yielded 1,100 patients who had undergone gastroenterostomy reconstruction (850 patients for B-II, 170 patients for R-Y, and 80 patients for Whipple's operation). After receiving gastroenterostomy reconstruction, 22 (2%) patients developed ALS. These patients all underwent contrast-enhanced abdominal MDCT. There were 12 men and 10 women (age range: 35-83 years, mean age±standard deviation: 64±13 years).
The 22 patients were subdivided into three groups according to prior surgery type: R-Y (n=9), B-II (n=7), and Whipple's operation (n=6). The clinical information, including acute abdominal symptoms, laboratory data, time between initial gastric operation and presentation of ALS, treatment, and prognosis of the three groups were recorded in the study. ALS was sometimes accompanied by pancreaticobiliary tract dilatation and infectious status in patients with the syndrome. The values of amylase, total bilirubin, alkaline phosphatase, white blood cell counts, and C-reactive protein were also evaluated.
MDCT was performed using a Brilliance 64 scanner (Philips Medical Systems, Cleveland, OH, USA). Ten patients were asked to drink 600 mL of 2.5% Gastrografin (Bayer Schering Pharma, Madrid, Spain) before MDCT scanning. Twelve patients did not take oral contrast. Each patient received 100 mL of nonionic contrast material [Ultravist 370 (iopromide); Bayer Schering Pharma AG, Berlin, Germany] was inserted into a cephalic or basilic vein through a 20-gauge catheter using a CT injector (Medrad, Pittsburgh, PA, USA) at a rate of 3 mL/s. MDCT was performed from the diaphragm to the symphysis pubis. We used the following image parameters for MDCT scanning and reconstruction: slice thickness, 5 mm; reconstruction thickness, 5 mm; reconstruction interval, 5 mm; beam pitch, 1.5; tube voltage, 120 kV; maximum tube current limited to 250 mA using dose modulation; and rotation time, 0.75 seconds. Scanning mode about imaging acquisition phases of all patients was not consistently performed at the time, but all patients had portal venous phase images (performed 60 s after contrast administration). Thus axial planes and reformatted coronal planes of portal venous phase were selected for our study.
We evaluated MDCT images as follows: the presence of bowel wall thickening at the anastomosis site and in the afferent or efferent loop; maximal diameter of the afferent loop (measured from outer wall to outer wall); presence of the keyboard sign; presence of the C-loop appearance, pancreaticobiliary tract dilatation, lymphadenopathy, ascites, peritoneal enhancement, or concurrent metastatic lesion.
Valvulae conniventes projecting into the fluid-filled bowel in the area of the transverse portion of the duodenum were designated as the keyboard sign.2 The fluid-filled dilated afferent loop shown on the coronal reformation was located at the mid-abdomen and the configuration of the afferent loop was shaped like a "C" character, so we called this picture the "C-loop appearance." Pancreaticobiliary tract dilatation was diagnosed when the maximal diameter of the common bile duct was larger than 8 mm and the greatest internal duct diameter of the pancreatic duct was larger than 5 mm at the head and 2 mm at the body and tail.2,4,5 Lymphadenopathy was considered metastatic if the short-axis diameter was larger than 10 mm.6 Ascites was classified as presence or absence.
The presumed causes of ALS on MDCT were classified as carcinomatosis, local recurrence, adhesions, or internal hernia. Carcinomatosis was suspected to be the cause when ascites and peritoneal enhancement were present and bowel thickening around the level of the obstruction was absent. When a tumor or focal bowel thickening in the surrounding tissues of the resected stomach was present, local recurrence was presumed to be the cause of obstruction.7 Adhesions were defined as a point of transition from a dilated bowel to a normal-caliber loop without a mass or other apparent cause.8 An internal hernia was diagnosed when crowding, stretching, and crossover of mesenteric vessels and the whirl sign were seen on MDCT.9
Two radiologists (Y.H.J. and W.C.C, with 5-10 years of experience in abdominal imaging) reviewed all abdominal radiographs, barium contrast studies, and abdominal MDCT, and identified the presumed causes of ALS in each patient. Conclusions were reached in consensus.
All surgical, pathological, and clinical evaluation findings were recorded. In the 12 patients who had undergone a second operation, the surgical findings were compared with the presumed cause of ALS on MDCT. In the other 10 patients, the cause of ALS was determined by endoscopic biopsy, abdominal tapping of ascites, or a clinical course. These patients were followed for at least one year until the acute symptoms subsided.
Statistical analyses were performed using statistical software (SPSS, version 14.0, Chicago, IL, USA; and SAS, version 9.0, Cary, NC, USA). Clinical information and laboratory data were analyzed using a one-way ANOVA test and are expressed as the mean±SD for continuous variables. Categorical variables and MDCT findings were analyzed by Pearson's χ2 test and are expressed as numbers of patients and percentages. A p value less than 0.05 was considered statistically significant.
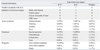
The clinical information for the three groups is displayed in Table 1. Twenty-two patients underwent subtotal gastrectomy with R-Y (n=9), subtotal gastrectomy with B-II (n=7), or Whipple's operation (n=6). Fifteen patients (68%) underwent gastroenterotomy surgery because of gastric cancer. The incidence of ALS in the above-mentioned gastric surgeries was about 5.3%, 0.8%, and 7.5%, respectively. The most common acute symptom, regardless of the type of previous surgery, was abdominal pain (82%) followed by vomiting. Secondary complications of ALS including pancreatitis (n=7; 32%), obstructive jaundice (n=5; 23%), and perforation (n=1; 4%) were observed. Twelve patients (55%) were treated with a second operation; most of them were patients with B-II. One patient died in the emergency department because of septic shock.
Comparisons of the clinical continuous variables are described in Table 2. There were no significant differences in age or laboratory data including increased levels of amylase, total bilirubin, alkaline phosphatase, white blood cell counts, or C-reactive protein among the three groups. The interval between the initial gastric operation date and the postoperative MDCT date was 2-96 months. The possibility of developing jejunal submucosal edema within a few days after operation was excluded from this study. The group with the longest mean time between previous surgery and presentation of ALS was that in which the patients had undergone B-II surgery (57 months; p<0.01).
The MDCT image findings are summarized in Table 3. Bowel wall thickening was evident in 13 patients and localized to the anastomosis in eight, who were confirmed to have local recurrence. The maximal diameter of the afferent loop ranged from 3.3 to 5.8 cm. We found that the keyboard sign and C-loop appearance was present in 21 (95%) and 22 patients (100%), respectively. The figure is an example of typical MDCT findings for ALS (Fig. 1). There was only one patient without presence of the keyboard sign (Fig. 2), due to bowel perforation. There was no statistical significance for other associated imaging findings, including the presence of common bile duct dilatation, pancreatic duct dilatation, and ascites.
The presumed causes of ALS on MDCT included carcinomatosis (n=8), local recurrence (n=8), adhesions (n=5), and internal hernia (n=1). In eight patients, carcinomatosis was confirmed by ascites cytology (n=7) or after a second operation (n=1), and these patients were treated using conservative (n=6) or surgical treatment (n=1) due to perforation of the afferent loop. One died before treatment. In eight patients, the recurrent tumor was diagnosed pathologically after a second operation (n=6) or an endoscopic biopsy (n=2), and these patients were managed by surgery (n=6) or chemotherapy (n=2). In five patients, the adhesions were matched by a second operation (n=4) or radiological findings along with clinical course (n=1), and these patients were managed with surgery (n=4) or conservative treatment (n=1). The internal hernia in the one remaining patient was confirmed and cured by a second operation.
The acute symptoms of 21 of the patients subsided after clinical management, except for one that died from septic shock before treatment in the emergency room. After searching a one-year follow-up of medical records, we found that no patient was readmitted because of recurrent ALS.
ALS is caused by three different mechanisms including mechanical obstruction of the afferent loop, preferential gastric emptying into the afferent loop, and obstruction of the efferent loop resulting in preferential filling of the afferent loop.1 This condition occurs infrequently following gastroenterotomy reconstruction. In 1971 Jordan10 reported the incidence of ALS as about 0.3%, but this data is from the distant past and may not match the actual current incidence rate. The overall incidence of ALS in our series was about 2%. This is probably because it has become more convenient for patients to seek medical advice and because of the easy availability of superior imaging modalities, such as MDCT, for diagnosing ALS, and because more patients who had undergone gastric surgery had postoperative follow-up CT at our institution.
The optimal reconstruction method for gastric cancer has not yet been universally established. There are both advantages and disadvantages to the different types of reconstruction. R-Y is better than the B-II in terms of the incidence of remnant gastritis and remnant gastric cancer, but it may be complicated by Roux stasis syndrome and a difficult endoscopic approach to the ampulla of Vater.11,12 In our study, 15 patients underwent gastroenterotomy reconstruction, R-Y (n=9) or B-II (n=6), because of gastric cancer. Comparison of the two groups showed that the B-II group had a lower incidence (0.8%) and a longer mean time before presenting ALS (57 months; p<0.01). This may be related to the non-physiological passage of food and occasional reversed peristalsis.12 If a patient with gastric cancer can be managed with either R-Y or B-II surgery, according to the results of our study, we consider the B-II operation to be a better choice than R-Y in terms of ALS complications.
Prior to the CT era, conventional UGI barium studies were used to assess ALS, but establishing a diagnosis by this means is problematic. Two classical findings were described for UGI barium studies. First, nonfilling of the afferent limb suggests ALS. Second, preferential filling and retention of barium in a dilated afferent limb for at least 60 minutes is consistent with ALS. However, 20% of normal afferent loops are not filled with a barium meal.13 Poor identification of the underlying cause of the ALS is another pitfall of UGI barium studies.
Currently, CT plays an important role in reliably establishing a diagnosis and is useful for determining the site, degree, and cause of ALS.1,2,14 Gayer, et al.1 demonstrated that a fluid-filled tubular structure containing small air bubbles in the right upper quadrant or crossing the midline on CT in symptomatic patients after gastroenterostomy is characteristic of a dilated afferent loop. However, in a few situations, ALS may be misrecognized as a pancreatic pseudocyst originating from the uncinate process on axial plane of CT images.15-17 On MDCT this may no longer pose a problem for the radiologist because this technology currently provides crucial improvements in the quality of two- and three-dimensional (3D) reformatted images, which can offer a coronal plane similar to the human anatomy with which they are familiar. Our study is the first regarding MDCT imaging for evaluation of ALS. We found that a fluid-filled C-shaped afferent loop (C-loop appearance) on coronal plane in combination with valvulae conniventes projecting into the lumen (keyboard sign) is the most common MDCT features of ALS. The presence of the keyboard sign in the dilated afferent loop may depend on the severity of obstruction. Merely in a rare condition (Fig. 2) with severe mechanical obstruction, the valvulae conniventes were effaced, and perforation of the afferent loop may occur due to the extremely high intraluminal pressure.
The curable causes of ALS such as adhesions or internal hernia or local recurrence should be identified by the radiologists. These causes might be properly managed by surgery. Conservative therapy has often been chosen for patients with ALS resulting from carcinomatosis because of their debilitated state or disseminated tumor. Kim, et al.2 demonstrated that the causes of afferent loop obstruction can be correctly predicted with axial plane CT in most cases. In our study, we believed that MDCT with coronal reformation is superior to axial plane CT in some situations, because problems related to partial volume effect on the axial plane are eliminated and the anatomical relationship of the organs is defined with easy compartmentalization of associated pathology (Fig. 3). Spatial resolution is better than with multiplanar reformatted images. With axial plane and coronal reformation images, we can predict curable or non-curable causes of ALS precisely and properly treat these patients by means of surgery, chemotherapy, or conservative management. However, in patients without peritoneal enhancement and ascites (non-disseminated carcinomatosis), preoperative identification of the etiology of ALS by MDCT is difficult in clinical practice.18
Our study has several potential limitations. First, this was a retrospective study with a relatively small number of patients, which limited its statistical power. Some patients may not present symptoms of ALS in the period we studied, and not all patients who had undergone gastroenterotomy underwent MDCT. We believe that further larger studies over a longer period would be able to more confidently address information regarding ALS. Second, we did not evaluate interobserver variability for evaluation of MDCT, which may have caused some degree of reporting bias. Third, although both observers were blinded to the final etiology, they were told that these patients had a diagnosis of ALS. Even if the benefits of adding coronal reformatted MDCT images in the interpretation process were not analyzed, we think coronal reformation is more helpful in diagnosing ALS because it makes sense intuitively and can display more bowel loops in a single image. Nevertheless, diagnostic performance for MDCT in our study still suggests that MDCT is a useful diagnostic imaging modality for evaluating patients following gastroenterotomy reconstruction.
Our study emphasizes the important advantages of MDCT in the evaluation of ALS. We found that a fluid-filled C-shaped afferent loop in combination with valvulae conniventes projecting into the lumen is the most common MDCT feature of ALS. MDCT can help radiologists to easily identify the site, level, and cause of ALS, and can help guide clinicians to properly treat these patients.
Figures and Tables
Fig. 1
Afferent loop obstruction in a 62-year-old man after Roux-en-Y gastroenterotomy. (A) Axial plane of MDCT shows a dilated fluid-filled afferent loop (arrow) located at the mid-abdomen and crossing between the aorta and superior mesenteric artery. (B) Coronal plane of MDCT reveals the configuration of the afferent loop to be of a "C" character (C). Keyboard sign (arrows) is also clearly demonstrated. Focal bowel thickening at the anastomostic region is present, suggesting local recurrence. Endoscopic biopsy confirmed the MDCT diagnosis of local recurrence.
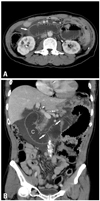
Fig. 2
Afferent loop obstruction in a 58-year-old man after Roux-en-Y gastroenterotomy. (A) Abdominal radiograph shows no remarkable findings. There is no evidence of intestinal obstruction or abnormal free extraluminal air accumulation. (B) Coronal plane of MDCT reveals perforation (arrow) of the dilated fluid-filled afferent loop. The C-loop appearance is well demonstrated, but the keyboard sign is not visualized. There is no focal bowel wall thickening or mass lesion at the anastomosis. Massive ascites and peritoneal enhancement are present, suggesting carcinomatosis. The second operation confirmed the MDCT diagnosis of carcinomatosis.
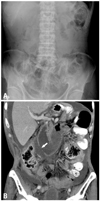
Fig. 3
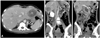
Afferent loop obstruction in a 35-year-old woman after Billroth-II gastrojejunostomy. (A) Axial plane of MDCT shows bowel wall thickening (arrow) instead of appreciable lobulated mass-like lesion at the anastomosis area. (M: liver metastasis) (B) Coronal plane of MDCT clearly demonstrates the lobulated contour of soft-tissue mass (arrows) at the anastomosis, suggesting local recurrence. (C) Another coronal plane of MDCT demonstrates the fluid-filled C-shaped afferent loop (C), in combination with valvulae conniventes projecting into the lumen (arrowheads). This MDCT finding is highly suggestive of bowel obstruction. The endoscopic biopsy confirmed the MDCT diagnosis of local recurrence inducing afferent loop syndrome. MDCT, multidetector-row computed tomography.
ACKNOWLEDGEMENTS
The study is granted by Tri-Service General Hospital (TSGH-C97-116).
The Institutional Review Board for Human Investigation of the Tri-Service General Hospital, National Defense Medical Center (TSGHIRB 098-05-002) approved this study.
References
1. Gayer G, Barsuk D, Hertz M, Apter S, Zissin R. CT diagnosis of afferent loop syndrome. Clin Radiol. 2002. 57:835–839.

2. Kim HC, Han JK, Kim KW, Kim YH, Yang HK, Kim SH, et al. Afferent loop obstruction after gastric cancer surgery: helical CT findings. Abdom Imaging. 2003. 28:624–630.

3. Yaghmai V, Nikolaidis P, Hammond NA, Petrovic B, Gore RM, Miller FH. Multidetector-row computed tomography diagnosis of small bowel obstruction: can coronal reformations replace axial images? Emerg Radiol. 2006. 13:69–72.

4. Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med. 2000. 19:727–730.

5. Berland LL, Lawson TL, Foley WD, Greenen JE, Stewart ET. Computed tomography of the normal and abnormal pancreatic duct: correlation with pancreatic ductography. Radiology. 1981. 141:715–724.

6. Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991. 180:319–322.

7. Ha HK, Kim HH, Kim HS, Lee MH, Kim KT, Shinn KS. Local recurrence after surgery for gastric carcinoma: CT findings. AJR Am J Roentgenol. 1993. 161:975–977.

8. Maglinte DD, Gage SN, Harmon BH, Kelvin FM, Hage JP, Chua GT, et al. Obstruction of the small intestine: accuracy and role of CT in diagnosis. Radiology. 1993. 188:61–64.

9. Blachar A, Federle MP, Dodson SF. Internal hernia: clinical and imaging findings in 17 patients with emphasis on CT criteria. Radiology. 2001. 218:68–74.

11. Iwahashi M, Nakamori M, Nakamura M, Naka T, Ojima T, Iida T, et al. Evaluation of double tract reconstruction after total gastrectomy in patients with gastric cancer: prospective randomized controlled trial. World J Surg. 2009. 33:1882–1888.

12. Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009. 39:647–651.

14. Zissin R, Hertz M, Paran H, Osadchy A, Gayer G. Computed tomographic features of afferent loop syndrome: pictorial essay. Can Assoc Radiol J. 2005. 56:72–78.
15. Beveridge CJ, Zammit-Maempel I. Afferent loop obstruction: CT appearances of an unusual cause of acute pancreatitis. Clin Radiol. 1999. 54:188–189.

16. Doherty M, Perret RS. Case 1: Afferent loop syndrome. AJR Am J Roentgenol. 1998. 171:852856–857.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download