Abstract
Purpose
In our previous study to investigate autonomic nervous system (ANS) activity due to radio frequency (RF) radiation using heart rate variability (HRV), drowsiness was observed in approximately half of all subjects. Therefore, the usage of HRV with unwanted drowsiness could falsely indicate the effects of RF radiation by mobile phones on the ANS. The objective of this study was to determine which posture is appropriate for accurate HRV analysis for provocation study.
Materials and Methods
A total of 52 healthy subjects (25 males and 27 females) participated in this experiment. We measured the number of times a subject showed drowsiness or sleep deprivation due to awakening, and analyzed HRV six times over 30 minutes in sitting and recumbent postures, using power spectrum.
Results
We employed the ratio of low frequency power to high frequency power (LFP/HFP) to analyze the changes in the ANS. The number of sleep deprivation occurrences in the sitting posture was significantly less than that in the recumbent posture (p<0.01), resulting in smaller increase of LFP/HFP. Although LFP/HFP of the two postures varied with time without any provocation, it was more stable in sitting than in recumbent postures.
Conclusion
A sitting posture is preferable to a recumbent posture for analyzing HRV, because of decreased drowsiness and sleep deprivation, thereby decreasing variation of LFP/HFP during experiment. Considering the drowsiness, it is also recommended that any experiment should be completed within 15 minutes, if possible.
The autonomic nervous system (ANS) regulates key functions, including the activity of cardiac muscles, smooth muscles, and glands. The ANS has two divisions: 1) the sympathetic nervous system, which accelerates heart rate, constricts blood vessels, and raises blood pressure; and 2) the parasympathetic nervous system, which slows the heart rate, increases intestinal and gland activity, and relaxes sphincter muscles.1 The ANS has been evaluated by heart rate variability (HRV), which utilizes the R-R interval on electrocardiograms (ECG). HRV is a good indicator for predicting adaptability against stress,2 investigating cardiovascular modulation with posture or sleep stage,3-5 and determining effects of provocation such as electromagnetic fields (EMF).6-9 However, HRV is very sensitive to age, stress, body mass index (BMI), menstruation, smoking, and other variables.2,3,5,8-12 In the evaluation of usefulness of HRV, the reproducibility of HRV has been found to be moderate-to-poor in studies examining intra-individual reproducibility.13 Therefore, careful discretion is needed to select subjects and experimental setups that minimize stress as much as possible when HRV is utilized to evaluate the ANS.
Most previous EMF provocation studies examined vital signs, questionnaires for subjective symptoms, or EMF perception in supine, sitting, or standing postures. However, there is still limited knowledge of the potential effect of EMF on cardiovascular function and the results are controversial. Experimental design to minimize possible confounding factors which affect HRV is very important. In our previous study, drowsiness was observed in approximately half of all subjects due to being in a recumbent posture for more than an hour in a quiet room.14 When any subject's drowsiness was noticed, the examiner made a noise to wake the subject and asked questions regarding subjective symptoms and EMF perception. We observed monotonically increased the ratio of low frequency power to high frequency power (LFP/HFP) at each exposure stage during a 30 minutes sham exposure in both electromagnetic hypersensitivity (EHS) and non-EHS groups. Zhong, et al.5 reported that sleep deprivation could increase LFP and LFP/HFP. Therefore, we concluded from our previous study that the usage of heart rate or HRV with sleep deprivation may falsely indicate the effects of RF radiation by mobile phones on the ANS. However, there have been few studies investigating the effects of drowsiness or sleep deprivation and posture on the LFP/HFP for provocation study. The results described herein led us to suggest a better posture, which results in less drowsiness or sleep deprivation, and thus, less increase in LFP/HFP during time course of provocation.
A total of 52 healthy subjects participated in this experiment: 25 males (25.4±2.9 years; 21.8±1.7 kg/m2) and 27 females (23.6±2.3 years; 20.4±2.0 kg/m2). They were recruited by advertisements at the Yonsei University Health System (YUHS). All subjects were non-smokers and asked not to do excessive exercise, use mobile phones more than 30 minutes, drink alcohol, or take any drugs at least 24 hours before the experiment. Female subjects did not participate in the experiments during their menstrual phases. As HRV is known to be dependent on age and obesity, the subjects were restricted to their twenties and a BMI of less than 22.9.10
The R-R intervals were acquired from 5 minutes of ECG data six times over 30 minutes in sitting and recumbent postures for each subject, and its power spectrum was obtained using data analysis software (TeleScan Ver.2.8, Laxtha, Daejeon, Korea). There are three peaks in the power spectrum of HRV: the first peak is the very low-frequency power (VLFP) that appears at less than 0.04 Hz; the second peak is the LFP that appears 0.04-0.15 Hz; and the third peak is the HFP that appears at 0.15-0.4 Hz. For VLFP, a clear conclusion has not yet been made. Accordingly, it has been excluded from most studies evaluating the ANS.14-16 LFP/HFP was obtained with HRV power spectrum to analyze balance between sympathetic and parasympathetic tone. A decrease in this score might indicate either increase in parasympathetic or decrease in sympathetic tone. To minimize individual difference in LFP/HFP, the resting LFP/HFP was set at 100%.
The lab was used exclusively for this experiment. To minimize interference including background EMF and noise, there was no other electrical equipment present. Before the experiment, each subject was made to rest on a chair (sitting posture) for one session, and on an experimental bed tilted up by 30° (recumbent posture) for another session for 10 minutes. The order of recumbent or sitting posture for a subject was randomly assigned to minimize experiment bias. No matter which came first, the second session was always given at approximately the same time of the day as the first session to maintain the subject's physiological rhythm. ECG was obtained with a PolyG-I (Laxtha) using Ag-AgCl electrodes (2223, 3M, St. Paul, MN, USA) placed on both arms and the right leg of participants.
The duration of each session was 40 minutes long, including 10 minutes of resting and six stages, with 5 minutes per stage. Thirty minutes was chosen based on previous short-term provocation studies in which subjects were exposed to EMF for approximately 30 minutes.6,7,9,14,17 Five minutes per stage was chosen because it is the minimum duration for HRV analysis.15 When the examiner noticed any subject's drowsiness, he made a noise to wake the subject up and counted the number of times drowsiness or sleep deprivation occurred during the 30 minutes.
Background extremely low frequency (ELF) and radio frequency (RF) fields in the lab were measured to ensure that the subjects were not influenced by the background fields. The average ELF electric and magnetic fields were 1.2±0.1 V/m (volts/meter) and 0.03±0.01 uT (micro Tesla), respectively, and measured using an ELF survey meter (HI-3604, Holaday, Minnetonka, MN, USA) and ELF gauss meter (EMDEX-II, ENERTECH, Campbell, CA, USA). The RF field was measured at 0.5±0.1 V/m with a frequency range from 824 to 849 MHz using a radiation meter (SRM 3000, Narda GmbH, Pfullingen, Germany). As the ambient temperature and relative humidity in the lab may affect ANS, a paired t-test was performed to check if there was any difference in temperature or humidity. There was no difference in temperature (p=0.640) or humidity (p=0.062) between the sessions of the sitting and recumbent postures (n=52).
The Mantel-Haenszel χ2 test was applied to determine the independency between sleep deprivation and posture. The Pearson χ2 test was used to analyze any difference in number of sleep deprivation and number of subject in each stage between sitting and recumbent postures. Repeated measures ANOVA were also performed to determine any difference in LFP/HFP between the postures. Post-hoc tests were performed to investigate any difference in LFP/HFP between each stage for each posture. SPSS software (SPSS10, SPSS Inc., Chicago, IL, USA) was used with a significance level of 0.05.
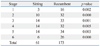
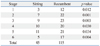
The Mantel-Haenszel χ2 test showed significant dependency of posture on the number of sleep deprivations (p<0.001) or number of sleep deprived subjects (p<0.001). Table 1 shows that the recumbent posture had a significantly higher number of sleep deprivation instances than did the sitting posture for every stage (p<0.01). Table 2 shows that the number of sleep deprived subjects was significantly more in the recumbent posture than that in the sitting posture for every stage (p<0.05). Since sleep deprivation was determined to be dependent on the posture, only the posture was assumed to be the independent variable in analyzing LFP/HFP.
Repeated measures ANOVA showed that LFP/HFP was significantly dependent on the posture (p=0.033). Fig. 1 shows mean±SE of LFP/HFP in sitting and recumbent postures for six stages with 52 subjects. It should be noted that LFP/HFP ratios in each stage of the sitting posture were smaller than those in each stage of the recumbent posture, suggesting that a sitting posture is better than a recumbent posture. This increased trend in LFP/HFP for the recumbent posture confirmed a previous report showing that sleep deprivation could increase LFP and LFP/HFP.5
For the sitting posture, there were significant differences in LFP/HFP between stages 1 and 4, stages 1 and 5, and stages 1 and 6 (p<0.01). The fact that there were no significant differences among the stages 1, 2, and 3 suggests that there were no changes in the ANS during the sitting posture for at least 15 minutes in this experimental setup. For the recumbent posture, there were significant differences in LFP/HFP between stage 1 and all the other stages (p<0.05). Therefore, these data suggest that the sitting posture is better than the recumbent posture, in terms of less increased LFP/HFP over time.
One disadvantage of the HRV method is that it is considerably influenced by stress, resulting in decreased HFP and increased LFP/HFP.15 Nam, et al.14 reported that LFP/HFP monotonically increased during 30 minutes of sham exposure in a recumbent posture for EHS and non-EHS groups. With an exception of stage 6, this monotonic increase is similar to that found in our previous result for a recumbent posture. At stage 6 of the recumbent posture, LFP/HFP did not show increase compared to stage 5. Even though the reason is not clear, the decreased LFP/HFP may be a reason for decreased number of sleep deprivation occurrences from 34 to 26 (Table 1). For these reasons, provocation studies should carefully be designed to control other factors, including posture, in order to minimize HRV variation.
Usage of heart rate or HRV with sleep deprivation may falsely indicate the effects of provocation, including EMF radiation, on the ANS. In this study, we confirmed that LFP/HFP significantly increased over time, compared to stage 1, due to sleep deprivation for the recumbent posture, but without any provocation. Therefore, any provocation study that requires waking for questionnaire should minimize stress as much as possible by limiting the experimental duration without weakening the experiment, as well as by minimizing sleep deprivation with a sitting posture. The fact that there were no significant differences in LFP/HFP between stages 1, 2, and 3 in a sitting posture suggests that there were no changes in ANS activity for up to 15 minutes in this experimental setup. In conclusion, sitting is preferred to a recumbent posture for provocation studies using HRV. It is also recommended that any experiment should be completed within 15 minutes, if possible, to avoid drowsiness.
Figures and Tables
 | Fig. 1Relative changes (%) in LFP/HFP of sitting and recumbent postures for each stage, which lasted for five minutes. The * and ** indicate statistically significant changes between stage 1 and other stages for sitting and recumbent postures. |
ACKNOWLEDGEMENTS
This work was supported by the Power Generation & Electricity Delivery of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant (No. 2009101030003B) funded by the Korean government (MKE) and also supported by a National Research Foundation (NRF) grant (2009-0083613) funded by the Korean government (MEST).
Notes
References
1. Gazzaniga MS, Ivry RB, Mangun GR. Gognitive neuroscience: The biology of the mind. 2002. 2nd ed. New York: Norton & Company.
2. Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000. 96:1–13.

3. Laitinen T, Niskanen L, Geelen G, Länsimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004. 96:2333–2340.

4. Park ES, Park CI, Cho SR, Lee JW, Kim EJ. Assessment of autonomic nervous system with analysis of heart rate variability in children with spastic cerebral palsy. Yonsei Med J. 2002. 43:65–72.

5. Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005. 98:2024–2032.

6. Wilén J, Johansson A, Kalezic N, Lyskov E, Sandström M. Psychophysiological tests and provocation of subjects with mobile phone related symptoms. Bioelectromagnetics. 2006. 27:204–214.

7. Parazzini M, Ravazzani P, Tognola G, Thuróczy G, Molnar FB, Sacchettini A, et al. Electromagnetic fields produced by GSM cellular phones and heart rate variability. Bioelectromagnetics. 2007. 28:122–129.

8. Buchheit M, Al Haddad H, Laursen PB, Ahmaidi S. Effect of body posture on postexercise parasympathetic reactivation in men. Exp Physiol. 2009. 94:795–804.

9. McNamee DA, Legros AG, Krewski DR, Wisenberg G, Prato FS, Thomas AW. A literature review: the cardiovascular effects of exposure to extremely low frequency electromagnetic fields. Int Arch Occup Environ Health. 2009. 82:919–933.

10. Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004. 93:381–385.

11. Hjortskov N, Rissén D, Blangsted AK, Fallentin N, Lundberg U, Søgaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur J Appl Physiol. 2004. 92:84–89.

12. Alyan O, Kacmaz F, Ozdemir O, Maden O, Topaloglu S, Ozbakir C, et al. Effects of cigarette smoking on heart rate variability and plasma N-terminal pro-B-type natriuretic peptide in healthy subjects: is there the relationship between both markers? Ann Noninvasive Electrocardiol. 2008. 13:137–144.

13. Højgaard MV, Holstein-Rathlou NH, Agner E, Kanters JK. Reproducibility of heart rate variability, blood pressure variability and baroreceptor sensitivity during rest and head-up tilt. Blood Press Monit. 2005. 10:19–24.

14. Nam KC, Lee JH, Noh HW, Cha EJ, Kim NH, Kim DW. Hypersensitivity to RF fields emitted from CDMA cellular phones: a provocation study. Bioelectromagnetics. 2009. 30:641–650.

15. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981. 213:220–222.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download