Abstract
Purpose
Tuberculous pleurisy is the most frequent extrapulmonary manifestation of tuberculosis. In spite of adequate treatment, pleural fibrosis is a common complication, but the mechanism has not been elucidated. This study is to determine whether epithelial to mesenchymal transition (EMT) of mesothelial cells occurs in tuberculous pleurisy.
Materials and Methods
Normal pleural mesothelial cells, isolated from irrigation fluids during operations for primary spontaneous pneumothorax, were characterized by immunofluorescence and reverse transcription polymerase chain reaction (RT-PCR). These cells were treated in vitro with various cytokines, which were produced in the effluents of tuberculous pleurisy. The isolated cells from the effluents of tuberculous pleurisy were analyzed by immunofluorescence and RT-PCR analysis.
Results
The isolated cells from the irrigation fluid of primary spontaneous pneumothorax had epithelial characteristics. These cells, with transforming growth factor-β1 and/or interleukin-1β treatment, underwent phenotypic transition from epithelial to mesenchymal cells, with the loss of epithelial morphology and reduction in cytokeratin and E-cadherin expression. Effluent analysis from tuberculous pleurisy using immunofluorescence and RT-PCR demonstrated two phenotypes that showed mesenchymal characteristics and both epithelial & mesencymal characteristics.
Tuberculous pleurisy is the most frequent extrapulmonary manifestation of tuberculosis.1 The pleura is a serous membrane that covers the lung parenchyma, mediastinum, diaphragm, and rib cages, and is divided into the visceral and parietal pleura. Both the visceral and parietal pleurae are lined with a single layer of flat mesothelial cells that have some similarity of epithelial cells. They are sensitive and responsive to various stimuli. Therefore, residual pleural thickening from 2 mm to 10 mm has been reported in 20% to 50% of all tuberculous pleurisy patients.2,3
Historically, pleural resident fibroblasts have been considered to be primary cells involved in the development of pleural fibrosis.4 However, emerging evidence from nephrology suggest that renal fibroblast and/or myofibroblasts can be derived from renal tubular epithelial cells in the process of tissue repair after injury through epithelial to mesenchymal transition (EMT).5-9 Other recent studies in dialysis patients also found the EMT process with peritoneal mesothelial cells.10-12
The most potent profibrotic cytokines, transforming growth factor (TGF)-β1 and interleukin (IL)-1β, have been considered main cytokines involved in pleural fibrosis of tuberculous pleurisy.13-17 Based on our finding that the mesothelial cells treated with TGF-β1 and/or IL-1β undergo a transition from an epithelial to a mesenchymal phenotype, we raised the possibility of EMT during fibrogenesis of tuberculous pleurisy. So far, there have been no studies testing our hypothesis. To address uncertainties surrounding our hypothesis, we used several approaches to investigate EMT in pleural mesothelial cells. We found that mesothelial cells with TGF-β1 and/or IL-1β treatment undergo a transition from an epithelial to a mesenchymal phenotype, raising the possibility of EMT during fibrogenesis. We then revealed evidence of EMT in effluents of tuberculous pleurisy by immunofluorescence and reverse transcription polymerase chain reaction (RT-PCR) studies. These studies provide evidence that EMT occurs during fibrogenesis in tuberculous pleurisy.
Recombinant human TGF-β1 and human IL-1β were used as the main cytokines. Monoclonal antibodies against E-cadherin (R&D systems, Minneapolis, MN, USA), pancytokeratin (Sigma Aldrich, St. Louis, MO, USA), vimentin (Sigma Aldrich, St. Louis, MO, USA), and rhodamine-phalloidin (Molecular probes, Inc., California, USA) were used for immunofluorescence studies.
In order to obtain normal pleural mesothelial cells, we collected the irrigation fluid of five primary spontaneous pneumothorax patients during bullectomy operations with video-assisted thoracoscopic surgery. To obtain the effluents of tuberculous pleury, thoracentesis from thirteen patients were done. If the pleural fluid adensine deaminase (ADA) level is above 40 U per L and the pleural fluid has a lymphocyte-to-neutrophil ratio greater than 0.75, a diagnosis of tuberculous pleurisy was made. Then, the cells were isolated by centrifugation and cultured until grown to confluence for 7-10 days. Finally, seven of the thirteen effluents reached confluences. The morphologic features of cells in confluent cultures were compared and remained stable during the two cell passages. All cells were cultured in Earle's M199 medium, 20 percent fetal-calf serum, 50 U of penicillin per milliliter, 50 µg of streptomycin per milliliter, and 2 percent Biogro-2 (containing insulin, transferrin, ethanolamine, and putrescine) (Biological Industries). For the first 24 to 48 hours of the culture with irrigation fluid of primary spontaneous pneumothorax patients, media were supplemented with 100 µg/mL cis-OH-proline (Sigma, St. Louis, MO, USA) to selectively eliminate fibroblasts from cultures. Mesothelial cells from spontaneous pneumothorax were treated with TGF-β1 at 0.5 ng/mL and/or IL-1β at 2 ng/mL for 72 hours. The concentration level of IL-1β used in this study was determined based on previous studies showing IL-1β induced EMT.10 Cells cultured from tuberculous pleurisy were stained after cell growth to confluence without cytokine supplement. For immunofluorescence studies, the cells were seeded on cover slips without Biogro-2. The procedures were performed in accordance with institutional guidelines, and were approved by an institutional review board. Informed consent was obtained from all study participants.
Total RNA was isolated using Trizol (Invitrogen, California, USA). The complementary DNA was obtained from 500 ng-1 µg of total RNA with the use of a random primer according to the manufacturer's instructions, the Superscript™ III First-Strand Synthesis system, and Platinum PCR SuperMix (Invitrogen, California, USA). Amplification of snail, E-cadherin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed according to the procedures described in a previous study.10
Immunofluorescence staining was used to detect E-cadherin, cytokeratin, vimentin, and F-actin in the cells from primary spontaneous pneumothorax and tuberculous pleurisy. Cells from effluents of tuberculous pleurisy and the Immunofluorescence studies were performed as described previously.10 The fluorescent images were obtained by confocal laser scanning microscope (Zeiss LSM 510, Oberkochen, Germany).
Cultured mesothelial cells from primary spontaneous pneumothorax had an almost round shape under the phase-contrast microscopy. The cells were stained strongly with epithelial markers, E-cadherin and cytokeratin. Mesenchymal markers vimentin and F-actin stained only some cortical areas of the cells. Our data ascertained that mesothelial cells from primary spontaneous pneumothorax were characterized by epithelial phenotype. After TGF-β1 treatment with 0.5 ng/mL, the round cell morphology gradually changed to elongated fibroblast-like cells under phase-contrast microscopy. Immunofluorescence microscopy demonstrated the loss of E-cadherin, cytokeratin with the replacement by vimentin, and the stress fiber reorganization by F-actin (Fig. 1). Our data suggested that TGF-β1 might induce the mesothelial cells to undergo EMT in vitro.
To determine whether IL-1β alone or in combination with TGF-β1 could induce EMT or have additive effects in EMT, cultured mesothelial cells from primary spontaneous pneumothorax were treated with IL-1β alone and in combination with 0.5 ng/mL concentration of TGF-β1. The treatment with IL-1β alone induced the cells to undergo morphologic changes to the elongated fibroblast-like cells under phase-contrast light microscopy (Fig. 1). The immunofluorescence stain after treatment with IL-1β alone also demonstrated the loss of E-cadherin, cytokeratin and replacement by vimentin and F-actin, although cytokeratin in some cells was still positive (Fig. 1). The treatment with TGF-β1 also induced morphologic changes to fibroblast-like cells, but the treatment IL-1β in combination with TGF-β1 did not reveal additional morphologic changes (Fig. 1).
The transcription factor snail is known as an inducer of EMT process. To determine whether the snail expression was associated with the phenotype changes during EMT, RT-PCR analysis was used. The expression of mRNA encoding E-cadherin was down-regulated with 0.5 ng/mL TGF-β1 treatments in cultured cells of primary spontaneous pneumothorax, and more down-regulation was detected with TGF-β1 treatment in combination with IL-1β (Fig. 2). While mRNA encoding snail was scarcely found without TGF-β1 treatment, the expression of mRNA encoding snail was revealed after 0.5 ng/mL TGF-β1 treatment. The combination of TGF-β1 with IL-1β tended to have an additional effect on snail expression in a patient with spontaneous pneumothorax (Fig. 2).
The cultured cells from tuberculous pleurisy revealed an expression of snail mRNA in all cases (Fig. 4), whereas the expression of E-cadherin mRNA was detected in only two cases (Fig. 4) (column 1 and 2), showing both characteristics of epithelial and mesenchymal phenotype in immunofluorescence stains (Fig. 3A and B). Our data suggest that EMT in mesothelial cells might be associated with the snail expression.
The mean age of seven patients with tuberculous pleurisy was 42.1 ± 15.6. No one had experienced any medical illness. On the chest radiographs or CT images, four patients had pulmonary tuberculosis with tuberculous pleurisy. Three of them showed remnant pleural thickening on their chest radiographs after six month treatment.
Cultured cells from the effluents of tuberculous pleurisy revealed two phenotypes. The first phenotype, which was found in five cases (Fig. 3C, D and E), demonstrated fibroblast-like cell characteristics that had elongated cell morphology, mesenchymal characteristics positive for vimentin, and stress fiber reorganization by F-actin. The second phenotype, which was found in two cases (Fig. 3A and B), had both characteristics of epithelial and mesenchymal cells, relatively round cell morphology, positive result for E-cadherin and cytokeratin, as well as stress fiber reorganization by F-actin under immunofluorescence study. In the second phenotype group, results of RT-PCR also revealed both expression of mRNA encoding E-cadherin and snail (Fig. 4) (column 1 and 2), whereas the first phenotype group (Fig. 4) (column 3-7) only revealed snail expression. Clinically, three patients with the first phenotype showed pleural thickening on their chest radiographs at the end of the treatment, but all patients with the second phenotype did not show remnant pleural thickening.
The results of immunofluorescence and RT-PCR studies indicated that some cases of tuberculous pleurisy have both characteristics of epithelial and mesenchymal cell. Therefore, our data suggest that EMT process might be involved in tuberculous pleurisy.
EMT is an essential process for embryonic fetal development. In adults, it is involved in tumor progression.18,19 Recently, EMT has been considered to be involved in tissue healing in response to various injuries because it serves as an additional source of myofibroblasts/fibroblasts, which are essential for tissue repair.8,20 One study demonstrated that renal tubular epithelium was transformed into more than one third of renal interstitial fibroblast via EMT using a model of gamma-glutamyl transferase lacZ transgenic mice.8
In this EMT process, TGF-β1 is a potent inducer of extracellular matrix formation. Actually, the TGF-β1 level was elevated in the effluents of tuberculous pleurisy and it was regarded as an important mediator of lung fibrogenesis.21,22 In pleural fibrosis, one potential role of TGF-β1 is to lead mesothelial cells to cause EMT. Our data showed that mesothelial cells treated with TGF-β1 went through EMT, which was typified by loss of epithelial markers such as cytokeratin or E-cadherin, gain of mesenchymal markers like vimentin, morphological change into myofibroblastic shape, and stress fiber reorganization with F-actin. Similarly, IL-1β has been known to lead renal epithelial cells to cause EMT, but some studies have failed to demonstrate that IL-1β induce other types of cells to undergo EMT.23,24
Therefore, we tested whether IL-1β could induce lung mesothelial cells to undergo EMT and have synergic effects in combination with TGF-β1. As a result, cultured mesothelial cells from pneumothorax treated with IL-1β alone showed similar changes in morphology and immunofluroscence stain compared with TGF-β1, although treatment with IL-1β alone showed weaker changes than those from treatment with TGF-β1. However, in combination with TGF-β1, additional morphologic changes were not obvious. We could not easily explain the reason, and quantitative methods would make it clear in the near future.
Among many different signaling pathways considered as initiators of EMT in various settings, all of them were eventually concluded in the loss of E-cadherin.19,25,26 E-cadherin is a significant component for the maintenance of the epithelial phenotype, so decreased expression of E-cadherin results from the loss of intercellular adhesion.20,27-29 Recent studies also demonstrate that suppression of E-cadherin expression, combined with the expression of transcription factor snail, induces EMT in carcinoma cells.30-34 Such studies have led to the speculation that E-cadherin is a potential epithelial master gene.
In our data, cultured cells from effluents of tuberculous pleurisy demonstrated two phenotypes. One phenotype, which was shown in five cases, revealed totally fibroblast-like cells under phase-contrast microscopy and immunofluorescence studies. Our results did not reveal where the cells originated from. However, in the remaining two cases, cultured cells revealed the characteristics of both epithelial and mesenchymal cells. Therefore, our finding could suggest that mesothelial cells undergo an epithleial to mesenchymal transition in the course of tuberculous pleurisy. Although it has been accepted that effluents from tuberculous pleurisy rarely contain more than 5% mesothelial cells,35,36 the reasons have not yet been determined. Based on the data of this study, there is the possibility that mesothelial cells change to fibroblast-like cells through EMT in the process of tuberculous pleurisy. Nevertheless, current methods of mesothelial cell isolation might not totally remove contaminating mesenchymal cells. So it is uncertain whether the factors associated with causing EMT may facilitate the growth of remnant mesenchymal cells and/or promote the loss of epithelial cells leading to increased mesenchymal marker expression. Therefore, we need more evidence to support EMT in tuberculous pleurisy.
In conclusion, our data suggest that profibrotic factors such as TGF-β1 and inflammatory cytokines such as IL-1β induce mesothelial cells to undergo EMT. These results will contribute to establish the pathophysiology of pleural fibrosis in tuberculous pleurisy. Furthermore, these data suggest new therapeutic targets that might ultimately prevent the fibrosis in the patients with tuberculous pleurisy.
Figures and Tables
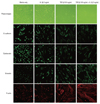 | Fig. 1Morphologic changes in mesothelial cells from the effluent of a patient with primary spontaneous pneumothorax with IL-1β ± TGF-β1 treatment. Cells obtained from the irrigation effluent of primary spontaneous pneumothorax during operation were treated with media only, IL-1β (2 ng/mL), TGF-β1 (0.5 ng/mL), and IL-1β + TGF-β1, respectively. Phase images are in the first row, and immunofluorescence stains in the remaining rows. Cultured cells after cytokines treatment were stained with monoclonal antibodies against E-cadherin, cytokeratin, vimentin, and F-actin. |
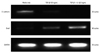 | Fig. 2Expression of snail transcription factor and the loss of E-cadherin expression in mesothelial cells with TGF-β1 ± IL-1β. Cells obtained from the irrigation effluent of a patient with primary spontaneous pneumothorax were treated with media only, TGF-β1 (0.5 ng/mL), and TGF-β1 + IL-1β (2 ng/mL), respectively. Cells were analyzed for snail and E-caherin messenger RNA expression by RT-PCR. RT-PCR, reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
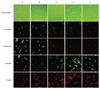 | Fig. 3Characteristics of cultured cells from effluents of tuberculous pleurisy. Cells characteristics from the effluents of five tuberculous pleurisy patients were observed under phase-contrast microscopy and stained with monoclonal antibodies against E-cadherin, cytokeratin, vimentin, and F-actin. |
 | Fig. 4Expression of messenger RNA encoding snail and E-cadherin in tuberculous pleurisy. Cells obtained from the seven effluents of tuberculous pleurisy were analyzed for snail and E-cadherin mRNA expression by RT-PCR. RT-PCR results of samples A) and B) from Fig. 3 are shown on columns 1 and 2 of Fig. 4, respectively. RT-PCR, reverse transcription polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
References
1. Seibert AF, Haynes J Jr, Middleton R, Bass JB Jr. Tuberculous pleural effusion. Twenty-year experience. Chest. 1991. 99:883–886.
2. de Pablo A, Villena V, Echave-Sustaeta J, Encuentra AL. Are pleural fluid parameters related to the development of residual pleural thickening in tuberculosis? Chest. 1997. 112:1293–1297.

4. Mutsaers SE, Prele CM, Brody AR, Idell S. Pathogenesis of pleural fibrosis. Respirology. 2004. 9:428–440.

5. Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, et al. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant. 2003. 18:1777–1784.

6. Nicolás FJ, Lehmann K, Warne PH, Hill CS, Downward J. Epithelial to mesenchymal transition in Madin-Darby canine kidney cells is accompanied by down-regulation of Smad3 expression, leading to resistance to transforming growth factor-beta-induced growth arrest. J Biol Chem. 2003. 278:3251–3256.

7. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001. 159:1465–1475.

8. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002. 110:341–350.

9. Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002. 61:1714–1728.

10. Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003. 348:403–413.

11. Selgas R, Bajo A, Jiménez-Heffernan JA, Sánchez-Tomero JA, Del Peso G, Aguilera A, et al. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant. 2006. 21:Suppl 2. ii2–ii7.

12. Aguilera A, Yáñez-Mó M, Selgas R, Sánchez-Madrid F, López-Cabrera M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr Opin Investig Drugs. 2005. 6:262–268.
13. Maeda J, Ueki N, Ohkawa T, Iwahashi N, Nakano T, Hada T, et al. Local production and localization of transforming growth factor-beta in tuberculous pleurisy. Clin Exp Immunol. 1993. 92:32–38.

14. Yanagawa H, Yano S, Haku T, Ohmoto Y, Sone S. Interleukin-1 receptor antagonist in pleural effusion due to inflammatory and malignant lung disease. Eur Respir J. 1996. 9:1211–1216.

15. Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J. 1997. 10:2411–2418.

16. Kunz CR, Jadus MR, Kukes GD, Kramer F, Nguyen VN, Sasse SA. Intrapleural injection of transforming growth factor-beta antibody inhibits pleural fibrosis in empyema. Chest. 2004. 126:1636–1644.

17. Ceyhan BB, Demiralp E, Karakurt ZL, Karakurt S, Sungur M. Transforming growth factor beta-1 level in pleural effusion. Respirology. 2003. 8:321–325.

18. Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003. 15:740–746.

20. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003. 9:964–968.

21. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, et al. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001. 56:907–915.

22. Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001. 163:1476–1483.

23. Vesey DA, Cheung CW, Cuttle L, Endre ZA, Gobé G, Johnson DW. Interleukin-1beta induces human proximal tubule cell injury, alpha-smooth muscle actin expression and fibronectin production. Kidney Int. 2002. 62:31–40.

24. Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis. 2001. 37:820–831.

25. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997. 390:465–471.

26. Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001. 98:6686–6691.

27. Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol. 2000. 1:91–100.
28. Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001. 105:425–431.
29. Pötter E, Bergwitz C, Brabant G. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocr Rev. 1999. 20:207–239.

30. Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000. 2:76–83.
31. Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000. 2:84–89.

32. Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol cell. 2001. 7:1267–1278.
33. Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002. 26:463–476.

34. Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995. 26:678–690.

35. Hurwitz S, Leiman G, Shapiro C. Mesothelial cells in pleural fluid: TB or not TB? S Afr Med J. 1980. 57:937–939.
36. Follador EC, Pimentel M, Barbas CS, Takagaki TY, Kairalla RA, Deheinzelin D, et al. [Tuberculous pleural effusion: clinical and laboratory evaluation]. Rev Hosp Clin Fac Med Sao Paulo. 1991. 46:176–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download