Abstract
Purpose
The molecular mechanisms that are responsible for the initiation and progression of breast cancers are largely unknown. This study was to analyze the cyclin B1, cdc2, p53 and p16 tumor suppressor genes in human breast cancer.
Materials and Methods
To investigate the role of cyclin B1, cdc2, p53 and p16 in the pathogenesis and progression of breast carcinomas, 98 cases of breast cancers were examined by immunohistochemical method. The correlations of cyclin B1, cdc2, p53 and p16 expression with various clinico-pathologic findings were analysed.
Results
In the normal breast tissues, cyclin B1, cdc2 and p16 were weakly expressed, while p53 was not expressed. On the other hand, cyclin B1, cdc2, p53 and p16 were overexpressed in breast cancer, showing correlation between the expression of cyclin B1 and cdc2 and breast cancers (p=0.00). The overexpressions of cdc2 and p16 were correlated with an infiltrative tumor border pattern and this was statistically significant (p<0.05). In addition, the overexpression of cdc2 was correlated with histologic high grade carcinomas (p=0.00).
Conclusion
Cyclin B1 and cdc2 appeared to be involved in the genesis or progression of breast cancers. In addition, the overexpressions of p16 and p53 may play important roles in more aggressive tumor and the overexpression of cdc2 is associated with progression of tumor to a higher grade of breast carcinomas. The deranged overexpressions of cyclin B1, cdc2, p16 and p53 may play an important role in human breast carcinogenesis.
Breast cancer is one of the most common types of cancer among women and it is the major cause of cancer death in the Korean population.1 Although the pathological staging classification of the disease is known to be the most important parameter that is related with the patients' clinical outcomes, clinico-pathological studies have shown that breast cancers are quite heterogenous, so that they reveal unpredictable biological behaviors. Therefore, it is necessary to find novel markers that are related to the biological behavior of breast tumors. Such trials have been done by using immunohistochemical or molecular biologic techniques.2-12
Because of the recent advances in understanding how the cell cycle is controlled and how it becomes deranged in cancer, various cell cycle regulators are now candidates as molecular markers. It has been shown that cell growth is tightly controlled by interactions of cyclins, cyclin dependent kinases (CDKs) and CDK inhibitors (CDKIs). Cyclins are proteins that govern progression through key checkpoints in the cell cycle and they act directly to regulate the activity of the central cell cycle kinase cdc2 and other related kinases.3-6 The dysregulated overexpression of cyclins appears to be involved in uncontrollable cell proliferation and early tumor development. Cyclin B1 regulates the G2-M transition of the cell cycle.13-15
Cdc2 is a protein that is expressed by the cdc2 gene. Cdc2 functions in conjunction with cyclin B1 to control mitosis.2 Regulation of cdc2 activity is a complex process that involves cyclin binding, subunit phosphorylations, CDKI binding and cyclin degradation. The cyclin/CDK complexes are known to function in regulating the cell-cycle. The cyclin/CDK complexes stimulate cell cycle progression, and the CDKIs induce cell cycle arrest by counteracting CDKs.3,14,15 The cyclin B1/cdc2 complex, which functions as a G2-M checkpoint, is essential for DNA mitosis. The cyclin B1/cdc2 complex functions in conjunction with CDKIs to control mitosis.2,7,15,16
The CDKIs identified in mammary cells are subdivided into two major classes. One family includes p15INK4B/MTS2, p16INK4A/MTS1 and p18. p27Kip1, p21WAF1/Cip1 and p57Kip2 form the other family of CDKIs, which are structurally unrelated to the INK4 family (p15INK4B/MTS2, p16INK4A/MTS1 and p18).10,11,17 The tumor suppressor protein p16 is a cyclin-dependent kinase inhibitor that negatively regulates cell proliferation by inhibiting the kinase activity of CDK4 and CDK6, and the tumor suppressor protein p16 subsequently promotes phosphorylation and inactivation of the tumor suppressor protein pRb. p16 is also a negative regulator of the mammarian cell cycle.10,11 Normal proliferating cells do not express significant levels of p16 prior to extensive rounds of cell division, which may suggest a late-stage, anti-proliferative role for p16 as in replicative cell senescence. p16 has broad specificity in vitro: it inhibits the kinase activity of the G1 cyclin complexes (Cyclin A-CDK2, Cyclin D-CDK4, and Cyclin E-CDK2) and, to lesser extent, the mitotic cdc2 and p16 do not associate with kinase subunits unless a cyclin is present. p16 regulation is an essential step in the pathway that links mitogenic signals to cell cycle progression.17
The p53 gene is located on the short arm of chromosome 17, and alterations in the p53 gene are the most common genetic lesions observed in human neoplasm.18 The functional derangement of the tumor suppressor gene p53 has been implicated as the main mechanism leading to the loss of cell-cycle control in human malignancies.19 The loss of wild-type p53 has been reported to be associated with human breast carcinomas.12
In this study, we examined the expressions of cyclin B1, cdc2, p16 and p53 in 98 cases of breast cancers by an immunohistochemical method, and analyzed the correlations of the expressions of cyclin B1, cdc2, p16 and p53 with various clinico-pathologic findings.
Ninety-eight cases of breast cancer that were histologically diagnosed from 2000 to 2008 at Kangbuk Samsung Hospital were included in this study. Also eighty-two cases of normal breast tissue which were taken from specimen after plastic surgery were included. The follow-up data of the cases were retrospectively retrieved from the clinical records of the hospital and the families of the patients. The classification, diagnoses and staging of breast cancers were done based on the AJCC Cancer staging manual of the American Joint Committee on Cancer.20 The histologic grade was determined by the Nottingham combined histologic grade (tubule formation, nuclear pleomorphism and mitotic count), assigning a value of 1 (favorable) to 3 (unfavorable) for each feature, and adding the scores together for all three categories.21
The study was strictly performed according to the Declaration of Helsinki and approved by the local Ethics Committee of the Kangbuk Samsung Hospital.
All of the tissues obtained from patients were routinely fixed in 10% buffered formalin and embedded in paraffin blocks. We produced the tissue microarray (TMA) blocks containing 2-mm diameter cores of the breast cancer tissues from the enrolled cases. One core was obtained from central portion of the mass on each case. The TMA blocks were sectioned at a 4 µm-thickness and were processed for immunohistochemistry. The slides were dehydrated, deparaffinated in xylene and then rehydrated in a graded series of alcohol solutions. The antibodies used were cyclin B1 (mouse monoclonal IgG1, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1 : 100), cdc2 (mouse monoclonal IgG2a, Santa Cruz Biotechnology, Santa Cruz, CA, USA 1 : 100), p16 (mouse anti Human E6H4, CINTEC, Heidelberg, Deutsch, 1 : 100) and p53 (monoclonal Rabbit Anti-Human 318-6-11, Dako, Glostrup, Denmark, 1 : 100). The immunostaining was performed with a compact polymer method (in a Bond Intense detection kit, Leica Biosystems, Newcastle, UK). Diaminobenzidine (DAB) was the chromogen and Meyer's hematoxylin was used for counterstaining. After three representative areas of the breast carcinomas were photographed using a digital camera under ×200 magnification, the numbers of all the cells and the positively stained cells were counted to assess the expressions of cyclin B1, cdc2, p16 and p53. The cytoplasmic and/or nuclear immunostainings were considered positive for cyclin B1, cdc2 and p16. Nuclear staining was considered positive on the immunostaining for p53. Positive staining in more than 10% of the tumor cells was considered positive. The cut off values in our study were defined previously,5,8,9 and we thought that 10% was optimal cut off value to evaluate statistical analyses. For the case of normal breast tissue, three representative areas were photographed under ×200 magnification in the mammary ductal epithelial areas. Clinical positive control cases for all the markers were included in every batch of staining. As a negative control, rabbit and mouse IgG isotypes were used instead of the primary antibodies.
The scores were entered into a Microsoft Excel spreadsheet. The scoring results were separated into two categories, either negative or positive. The uninterpretable results were eliminated from further consideration.
The relationships between the expressions of cyclin B1, cdc2, p16 and p53 and the various clinicopathological findings were evaluated using the Fisher exact test and the Pearson Chi-square test. The Kaplan-Meier survival curves were generated to access whether any level of cyclin B1, cdc2, p16 and p53 had any effect on the overall survival of patients with breast cancer and the resulting curves were compared using the log-rank test. p values less than 0.05 were considered to be statistically significant. The statistical analyses were performed with PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
All 98 cases were women and their age ranged from 28 to 79 years, with a mean age of 50.1±10.2 years. The median follow-up period was 80 months (range: 13 months to 118 months). The survival of patients was grouped as no evidence of disease (NED)(n=80), dead of disease (DOD)(n=9) and alive with disease (AWD)(n=9). There were 29 cases of stage I, 35 cases of stage IIa, 14 cases of stage IIb, 11 cases of stage IIIa, 1 case of stage IIIb and 8 cases of stage IIIc. The histologic subtypes included invasive ductal carcinoma (IDC)(n=91) and others (n=7).
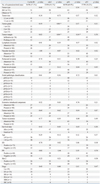
The clinico-pathologic parameters included tumor type, tumor size, tumor grade, tumor margin, lymphatic invasion, vascular invasion, perineural invasion, nodal involvement, nodal pathologic classification, extensive intraductal component, tumor necrosis, tumor recurrence, survival state, age, estrogen receptor, progesterone receptor, her-2 and stage. The clinical data of the patients and the relationships between the clinico-pathologic parameters and the cyclin B1, cdc2, p16 and p53 expressions in 98 human breast carcinomas are summarized in Table 1. Two pathologists independently examined the cases and, if there was disagreement, we had a mutual agreement with an another pathologist. However, there was trivial interobserver variations on interpretation. Cyclin B1, cdc2, p16 and p53 were diffusely expressed in 56 cases (57.1%), 53 cases (54.1%), 68 cases (69.4%) and 57 cases (58.2%), respectively, out of 98 cases with breast cancers. The mean labeling indices of cyclin B1 and cdc2 were 31.8% and 38.3%, respectively. The immunostaining of cyclin B1 was predominantly detected in the cytoplasm, and cdc2 staining was mainly detected in the cytoplasm and to lesser extent in the nuclei. In the normal breast tissue, cyclin B1 and cdc2 were weakly expressed in the cytoplasm (Fig. 1A-D). Other cells, such as lymphocytes, monocytes and macrophages, did not show cyclin B1 and cdc2 immunostains. The mean labeling indices of cyclin B1 and cdc2 in the glandular epithelial cells of the normal breast tissues were 5% and 10%, respectively, and these findings were statistically significant (p<0.05). The immunostaining of p16 was detected in the nuclei and cytoplasm (Fig. 1E), and p53 was predominantly detected in the nuclei (Fig. 1F). In the normal breast tissue, p16 was weakly expressed in the nuclei and cytoplasm, but p53 was not expressed. The cdc2 and p16 expressions were 69.8% and 72.1% in the cases with an infiltrative tumor border, whereas 30.2% and 27.9% in the cases with a pushing tumor border, respectively, and these findings were statistically significant (p<0.05). In addition, cdc2 expression was seen in 21.7% of the grade 1 cases, in 60.5% of the grade 2 cases and in 67.6% of the grade 3 cases, and these findings were statistically significant (p<0.05).
The cyclin B1 expression showed a tendency to increase according to that of cdc2, and it was statistically significant (p=0.00). However, the correlations between the expressions of cyclin B1 and p16 and between the expressions of cyclin B1 and p53 were not statistically significant (Table 2). The correlations between the expressions of cdc2 and p16 and between the expressions of cdc2 and p53 were not statistically significant (Table 3). Furthermore, the correlation between the expressions of p53 and p16 was not statistically significant (Table 4).
The survival rate was calculated for the 98 patients, and the mean observation duration was 81.7±4.5 months. There was no significant difference in survival according to the expressions of cyclin B1, cdc2, p16 and p53, regardless of the cut-off value for the Kaplan-Meier survival curves (Fig. 2). There was no influence on the overall survival according to the expressions of cyclin B1, cdc2, p16 and p53.
In the present study, cyclin B1 was found to be diffusely expressed in 57.1% of the cases. It has generally been reported in the literature5 that cyclin B1 is overexpressed at a relatively high frequency (42%) when detected by an immunohistochemical method, and it has been found to correlate with various clinico-pathologic parameters including high tumor grade and distant metastasis. In our study, cyclin B1 overexpression was detected in breast cancers, similar to earlier studies,3,5,6 however, it was not correlated with any clinico-pathologic parameters, unlike the results of the previous studies.3,5 Therefore, further follow up cohort study is needed.
In our present study, cdc2 was overexpressed in 54.1% of the cases and this was associated with a higher histologic grade and infiltrative growth pattern. Some studies found that a cdc2 overexpression occurs at a high frequency (66%) and it is associated with a higher histologic grade and a p53 expression.8 Our results are in partial agreement with those of Kourea and co-workers,8 who noted a positive association of the tumor grade with a cdc2 overexpression. These findings probably reflect a measure of proliferative cellular activity.22
In this immunohistochemical study, the mean labeling indices of cyclin B1 and cdc2 in breast carcinomas were 31.8% and 38.3%, respectively, and those of cyclin B1 and cdc2 in the mammary ductal cells of normal breast tissues were 5% and 10%, respectively, showing much lower than those of the breast carcinomas. There was quite different staining patterns between breast carcinomas and normal breast tissues: In the normal breast tissue, there was a multifocal staining pattern of cyclin B1 and cdc2. In contrast, however, cyclin B1 and cdc2 were diffusely expressed in the breast carcinomas. The reason why the labeling index of cdc2 is higher than that of cyclin B1 in both normal breast tissue and cancer breast tissues is not definite. But the fact that cdc2 is present during all phases of cell cycle, except G0 phase, however cyclin B1 is expressed in the later phases of cell cycle and re-synthesized on the beginning of S phase may be the reason of those results.23 In our study, the correlation between the overexpressions of cyclin B1 and cdc2 was noted, and it was statistically significant. These results suggest that cyclin B1 and cdc2 cooperate closely to play a role in the progression or genesis of breast carcinomas. During interphase, cyclin B1 is initially localized in the cytoplasm due to the function of the cytoplasmic retention sequence. Then, most of the cyclin B1-cdc2 complex are rapidly translocated from the cytoplasm to the nucleus in late prophase. Therefore, the cyclin B1 and cdc2 overexpressions may be caused by unlimited protein synthesis, impaired proteolytic degradation and due to some other reasons.14,16 The mechanism of how cyclin B1 and cdc2 participate in tumor progression is not clear. p53 inactivates cdc2 kinase, which controls the G2 checkpoint in the cell cycle. This cdc2 kinase inactivation results from the repression of cyclin B1 and cdc2 transcription. The enforced expression of both cyclin B1 and cdc2 leads to overriding p53-mediated G2-M arrest. The constitutive activation of cyclin B1-associated cdc2 kinase overrides p53-mediated cell cycle arrest, so that the cell cycle can continue without the G2 checkpoint in cases where cyclin B1 is overexpressed. Nevertheless, it remains to be determined how the overexpressions of cyclin B1 and cdc2 are involved in oncogenesis and tumor progression.
The overexpressions of cyclin B1 and cdc2 have been shown to be an important factor affecting survival in several malignant diseases, including breast cancer,3 esophageal squamous cell carcinoma,23 non-small cell carcinoma24 and hepatocellular carcinoma.25 In the present study, we found that the expressions of cyclin B1 and cdc2 had no influence on survival, according to the Kaplan-Meier survival curve analysis, regardless of the cut-off value.
To gain a better understanding of the molecular changes underlying breast carcinoma's potentially aggressive behavior, we studied the immunoreactivity for p16 and p53 in breast carcinomas and normal breast tissue of human specimens, and found that p16 was frequently overexpressed (69.4%) in breast cancers and associated with an infiltrative tumor border. Some other studies have revealed down-regulation of p16 in breast carcinoma, a change that is usually accompanied with an overexpression of p16 immunoreactivity and aggressive behavior.9-11,17,26,27 Overexpression of p16 protein has been found in many types of human malignancy, such as breast,9 gastric26 and ovarian cancer.27 In addition, previous studies have also demonstrated that p16 overexpression is associated with tumor progression and a poor prognosis for different tumors such as breast,9 prostate28 and ovarian cancer.29 However, normal human tissues display low or undetectable levels of p16 protein.30 The high levels of p16 found in breast cancer cells suggest that this protein may play a role in tumor development and progression. Many studies have characterized p16 as an independent prognostic factor in various human cancers, including breast carcinomas.3,10,17,26-29 These observations together, suggest that p16 might be used as a marker for tumor progression and prognosis, as well as for diagnosis of tumor. Moreover, p16 seems to be able to promote apoptosis in human breast carcinoma cells and it may be instrumental in the regression of breast carcinomas through mechanisms that are currently unknown.
The functional derangement of the tumor suppressor gene p53 has been implicated as the main mechanism leading to the loss of cell-cycle control in human breast cancer.31-33 In this study, p53 overexpression was found to be not associated with any clinico-pathologic parameters, in contrast to previous studies.12,18,19,30-32 Therefore, further large scale cohort study is required.
In conclusion, the overexpressions of cyclin B1, cdc2, p16 and p53 are important in the development and progression of human breast carcinomas. In addition, evidences indicate that the overexpressions of p16 and p53 may play important roles in more aggressive tumor, and that the overexpression of cdc2 is associated with progression of tumor to a higher grade of breast carcinomas. Significant difference in the cyclin B1 and cdc2 values between normal mammary ductal epithelial cells and breast carcinomas indicates a consequential role for the overexpressions of cyclin B1 and cdc2 in the malignant transformation of breast epithelial cells. Future studies to examine resected human breast tumor samples are required to better delineate effectors that mediate the malignant phenotype. This is essential in order to better understand the mechanisms that regulate breast carcinogenesis.
Figures and Tables
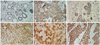 | Fig. 1Immunostainings for cyclin B1 and cdc2 in normal breast tissue and invasive ductal carcinoma. Cyclin B1 is weakly and multifocally highlighted in the cytoplasm of normal mammary ductal cells (A), whereas carcinoma cells diffusely express cyclin B1 in the cytoplasm (B). cdc2 is weakly and multifocally highlighted in the cytoplasm of normal mammary ductal cells (C), whereas it was detected mainly in the cytoplasm and to lesser extent in the nuclei (D) of carcinoma cells. p16 (E) is diffusely detected in the nuclei and cytoplasm (E) and p53 (F) is strongly expressed in the nuclei of invasive ductal carcinoma cells. |
 | Fig. 2Kaplan-Meier survival curve stratified according to the extent of cyclin B1 (A), cdc2 (B), p16 (C) and p53 (D). When the expression of cyclin B1 was stratified as 10% or over (n=56) and below 10% (n=42), the survival curve of cyclin B1 shows no significance (A). When the expression of cdc2 was stratified as 10% or over (n=53) and below 10% (n=45), the survival curve of cdc2 shows no significance (B). When the expression of p16 was stratified as 10% or over (n=68) and below 10% (n=30), the survival curve of p16 shows no significance (C). When the expression of p53 was stratified as 10% or more (n=57) and below
10% (n=41), the survival curve of p53 shows no significance (D). |
Table 1
Association between the Clinico-Pathologic Parameters and the Cyclin B1, cdc2, p16 and p53 Expressions in 98 Human
Breast Carcinomas
ACKNOWLEDGEMENTS
This study was supported by the Hyosuk Research Fund from Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine.
References
1. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.

2. Ohta T, Okamoto K, Isohashi F, Shibata K, Fukuda M, Yamaguchi S, et al. T-loop deletion of CDC2 from breast cancer tissues eliminates binding to cyclin B1 and cyclin-dependent kinase inhibitor p21. Cancer Res. 1998. 58:1095–1098.
3. Aaltonen K, Amini RM, Heikkilä P, Aittomäki K, Tamminen A, Nevanlinna H, et al. High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer. 2009. 100:1055–1060.

4. Agarwal R, Gonzalez-Angulo AM, Myhre S, Carey M, Lee JS, Overgaard J, et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin Cancer Res. 2009. 15:3654–3662.

5. Suzuki T, Urano T, Miki Y, Moriya T, Akahira J, Ishida T, et al. Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2007. 98:644–651.

6. Yuan J, Yan R, Krämer A, Eckerdt F, Roller M, Kaufmann M, et al. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene. 2004. 23:5843–5852.

7. Kim SJ, Nakayama S, Miyoshi Y, Taguchi T, Tamaki Y, Matsushima T, et al. Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol. 2008. 19:68–72.

8. Kourea HP, Koutras AK, Scopa CD, Marangos MN, Tzoracoeleftherakis E, Koukouras D, et al. Expression of the cell cycle regulatory proteins p34cdc2, p21waf1, and p53 in node negative invasive ductal breast carcinoma. Mol Pathol. 2003. 56:328–335.

9. Dublin EA, Patel NK, Gillett CE, Smith P, Peters G, Barnes DM. Retinoblastoma and p16 proteins in mammary carcinoma: their relationship to cyclin D1 and histopathological parameters. Int J Cancer. 1998. 79:71–75.

10. Vallian S, Sedaghat M, Nassiri I, Frazmand A. Methylation status of p16 INK4A tumor suppressor gene in Iranian patients with sporadic breast cancer. J Cancer Res Clin Oncol. 2009. 135:991–996.

11. Zhang J, Pickering CR, Holst CR, Gauthier ML, Tlsty TD. p16INK4a modulates p53 in primary human mammary epithelial cells. Cancer Res. 2006. 66:10325–10331.

12. Iwaya K, Tsuda H, Hiraide H, Tamaki K, Tamakuma S, Fukutomi T, et al. Nuclear p53 immunoreaction associated with poor prognosis of breast cancer. Jpn J Cancer Res. 1991. 82:835–840.

15. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995. 9:1149–1163.

16. Porter LA, Donoghue DJ. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog Cell Cycle Res. 2003. 5:335–347.
17. Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006. 16:1105–1110.

20. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 2010. 7th ed. New York: Springer-Verlag;347–376.
21. Harris L, Fritsche H, Mennel R, Norton L, Ravdin p, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007. 25:5287–5312.

22. Gannon JV, Nebreda A, Goodger NM, Morgan PR, Hunt T. A measure of the mitotic index: studies of the abundance and half-life of p34cdc2 in cultured cells and normal and neoplastic tissues. Genes Cells. 1998. 3:17–27.

23. Murakami H, Furihata M, Ohtsuki Y, Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch. 1999. 434:153–158.

24. Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000. 60:4000–4004.
25. Ito Y, Takeda T, Sakon M, Monden M, Tsujimoto M, Matsuura N. Expression and prognostic role of cyclin-dependent kinase 1 (cdc2) in hepatocellular carcinoma. Oncology. 2000. 59:68–74.

26. Feakins RM, Nickols CD, Bidd H, Walton SJ. Abnormal expression of pRb, p16, and cyclin D1 in gastric adenocarcinoma and its lymph node metastases: relationship with pathological features and survival. Hum Pathol. 2003. 34:1276–1282.

27. Lee CT, Capodieci P, Osman I, Fazzari M, Ferrara J, Scher HI, et al. Overexpression of the cyclin-dependent kinase inhibitor p16 is associated with tumor recurrence in human prostate cancer. Clin Cancer Res. 1999. 5:977–983.
28. Halvorsen OJ, Høstmark J, Haukaas S, Høisaeter PA, Akslen LA. Prognostic significance of p16 and CDK4 proteins in localized prostate carcinoma. Cancer. 2000. 88:416–424.

29. Dong Y, Walsh MD, McGuckin MA, Gabrielli BG, Cummings MC, Wright RG, et al. Increased expression of cyclin-dependent kinase inhibitor 2 (CDKN2A) gene product P16INK4A in ovarian cancer is associated with progression and unfavourable prognosis. Int J Cancer. 1997. 74:57–63.

30. Gonzalez-Zulueta M, Bender CM, Yang AS, Nguyen T, Beart RW, Van Tornout JM, et al. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995. 55:4531–4535.
31. Bartley AN, Ross DW. Validation of p53 immunohistochemistry as a prognostic factor in breast cancer in clinical practice. Arch Pathol Lab Med. 2002. 126:456–458.

32. Wegman PP, Marcus NJ, Malakkaran BP, Wingren S. Biological significance of allele specific loss of the p53 gene in breast carcinomas. Breast Cancer Res Treat. 2009. 118:15–20.

33. O'Hanlon DM, Kiely M, MacConmara M, Al-Azzawi R, Connolly Y, Jeffers M, et al. An immunohistochemical study of p21 and p53 expression in primary node-positive breast carcinoma. Eur J Surg Oncol. 2002. 28:103–107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download