Abstract
Purpose
Stimulation of human aortic smooth muscle cells (hAoSMCs) with native low-density lipoprotein (nLDL) induced the production of interleukin-8 (IL-8) that is involved in the pathogenesis of cardiovascular diseases. However, the process of signal transduction of nLDL was currently uncharacterized. Therefore, the aim of this study was to investigate the signal transduction pathway of nLDL-dependent IL-8 production and the effect of IL-8 on hAoSMCs migration.
Materials and Methods
nLDL was prepared by ultracentrifugation with density-adjusted human serum of normocholesterolemia. In hAoSMCs, IL-8 secreted to medium was measured using ELISA assay, and Western blot analysis was performed to detect p38 MAPK activation as a key regulator of IL-8 production. nLDL-dependent H2O2 generation was determined by microscopic analysis using 2',7'-dichlorofluoroscein diacetate (DCF-DA). IL-8-induced migration of hAoSMCs was evaluated by counting the cell numbers moved to lower chamber using Transwell plates.
Results
nLDL-induced IL-8 production was completely blocked by preincubation of hAoSMCs with pertussis toxin (PTX), which inhibited nLDL-dependent p38 MAPK phosphorylation. PTX-sensitive G-protein coupled receptor was responsible for nLDL-dependent H2O2 generation that was abrogated with preincubation of the cells with of polyethylene glycol-conjugated catalase (PEG-Cat). Pretreatment of PEG-Cat prevented nLDL-induced p38 MAPK phosphorylation and IL-8 production, which was partly mimicked by treatment with exogenous H2O2. Finally, IL-8 increased hAoSMCs migration that was completely blocked by incubation with IL-8 neutralizing antibody.
Elevated plasma level of native low-density lipoprotein (nLDL) contributes to numerous cardiovascular diseases including hypercholesterolemia, atherosclerosis, hypertension, heart failure, and diabetes, all of which are closely linked to vascular migration and proliferation1-3 and oxidative stress.4 It has been demonstrated that the in vitro observations of nLDL stimulation on vascular cells closely resemble many of the early changes observed both in vivo and ex vivo in human and animals during hypercholesterolemia.5-8
Reactive oxygen species (ROS) have deleterious effects on cardiovascular function.9 In nLDL-and oxidized LDL-stimulated vascular smooth muscle cells (VSMCs), O2-contributes to cell proliferation, which is particularly important to the pathophysiological conditions of in-stent restenosis, transplant vasculopathy, and vein bypass graft failure. Furthermore, pretreatment with superoxide scavengers and superoxide dismutase completely blocks the cell proliferation,10,11 implying that O2- is involved in nLDL-induced cell proliferation. On the other hand, intracellular H2O2 may mediate the mitogenic activity of growth factors through inhibition of protein tyrosine phosphatases.12 These observations suggest that ROS such as O2- and H2O2 play different physiological roles in VSMCs. Therefore, the pathophysiological role of nLDL-dependent H2O2 in VSMCs has to be elucidated.
Increased production of IL-8 as an C-X-C chemokine is involved in the recruitment of mononuclear leukocytes and stimulates the formation of atherosclerotic plaques made by macrophages, endothelial cells, and SMCs.13,14 Furthermore, VSMCs migration is associated with progression of atherosclerosis through the invasion into the intima where the plaque is formed.15 We previously reported that nLDL stimulation increased IL-8 production in human aortic SMCs (hAoSMCs) via phosphorylation of p38 MAPK and activation of transcriptional factor activator protein-1 (AP-1) with NF-kB participation. However, little information exists on nLDL-dependent signal transduction for the production of IL-8 in hAoSMCs.
Therefore, we examined the nature of the receptor and ROS responsible for nLDL-induced IL-8 production and the pathophysiological role of IL-8 played hAoSMCs migration.
Cholera toxin, pertussis toxin, heparin, and genistein were purchased from Calbiochem. All other reagents, such as H2O2 and polyethylene glycol conjugated-catalase (PEG-Cat), were purchased from Sigma unless otherwise stated.
hAoSMCs were purchased from Clonetics and cultured in SmGM-2 Bullet kit medium (Clonetics) in 5% CO2 at 37℃. Twenty-four hours before experiments, growth medium was removed and replaced with DMEM medium (Gibco) containing 0.1% FBS (Gibco) for 24 hours (maintenance medium).
n-LDL (density 1.019-1.063 g/mL) was isolated from the plasma of normocholesterolemic subjects (serum cholesterol <6.2 mmol/L) by differential ultracentrifugation as previously described.16 During LDL manipulation and storage, particular precautions were taken in order to maintain nLDL integrity and prevent oxidation. The content of endotoxin was below the detection limit (<1 ng/mL), as measured using an endotoxin assay kit (Pharmingen). Prepared nLDL was confirmed by thiobarbituric acid reactive substances (TBARS) assay measurement using malondialdehyde as a standard and compared its oxidation with commercial nLDL and Ox-LDL (Intracel Co., Frederick, MD, USA).
Pretreated cells grown on gelatin-coated glass were stained with DCF-DA (2',7'-dichlorofluoroscein diacetate, 10 µmol/L), followed by incubation at 37℃ for 30 minutes. Cells were washed three times with phosphate-buffered saline (PBS) and imaged at 488/535 nm using an epifluorescence microscope (Olympus BX51).
The amounts of IL-8 released into the media were assayed using a human IL-8 ELISA Kit II (OptEIA, BD Biosciences, NJ, USA) according to the manufacturer's instructions.
Cells treated with different chemicals were lysed in SDS sample buffer (62.5 mmol/L Tris pH6.8, 2% SDS, and 10% glycerol). Each sample was resolved on a 10% SDS-PAGE, transferred onto PVDF membranes (Pall), analyzed with antibodies according to the supplier's instructions, and then visualized with peroxidase and an enhanced-chemiluminescence system (ECL kit, Amersham, NJ, USA). Polyclonal phospho-p38 MAPK was purchased from Cell Signaling Technology and normalization was performed with the smooth muscle cell (SMC)-specific α-actin antibody (BD Biosciences, NJ, USA).
Transwell plate (13 mm gelatin-coated polycarbonate filter, 8 µm pore size, Costar Corp.) was used for the migration assay. Briefly, suspended cells (1×105) were added to the upper chamber of the Transwell insert, and IL-8 or neutralizing antiserum against IL-8 (R&D system, MN, USA) was mixed with the medium [DMEM+1% FBS+0.1% bovine serum albumin (BSA)] in the lower chamber. After the transwell was incubated for 8 hours, the filters were fixed with methanol (10 minutes at 4℃) and stained with hematoxylin. The number of migrated cells was determined by microscopy (Olympus BX51, 400X high power field).
First, we tested whether prepared LDL underwent any change such as oxidative modification. However, LDLs prepared in 3 independent experiments showed no oxidation, because the value was -0.60±0.06 µmol/L in TBARS assay. This value was comparable to commercially available nLDL, which was -0.55±0.04 µmol/L, whereas, oxidized LDL was 7.95±0.60 µmol/L.
Since we previously demonstrated that stimulation of hAoSMCs with nLDL increased IL-8 production via p38 MAPK activation 16, we attempted to determine the nature of the receptor responsible for IL-8 production. As shown in Fig. 1A, increased IL-8 production by nLDL from 4.96±0.36 ng/mL to 14.03±0.56 ng/mL (Fig. 1A, *vs. untreated, p<0.01) was completely blocked by pretreatment of the cells with PTX (Fig 1A, **vs. nLDL=3.23±0.03 ng/mL vs. 14.03±0.56 ng/mL, p<0.01). This effect of pertussis toxin (PTX) was associated with inhibition of p38 MAPK phosphorylation (Fig. 1B). On the other hand, cholera toxin (CTX) treatment had no effect on IL-8 production (Fig. 1A, #vs. nLDL=13.63±0.39 ng/mL vs. 14.03±0.56 ng/mL, not significant) and p38 MAPK phosphorylation (Fig. 1B). Furthermore, inhibitors related to receptor-associated signal transduction, such as heparin for the classical nLDL receptor or genistein for receptor tyrosine kinase, were employed, however, these agents had no inhibitory effect on IL-8 production (Fig. 1A, nLDL+heparin vs. nLDL=13.91±0.84 ng/mL vs. 14.09±1.38 ng/mL, not significant; nLDL+genistein vs. nLDL=13.89±0.40 ng/mL vs. 14.09±1.38 ng/mL, not significant) and p38 MAPK activation (Fig. 1C). In addition to nLDL, sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) have been known as agonists binding to Gi-protein coupled receptors. Therefore, nLDL was compared with S1P and LPA in terms of IL-8 production. Unlike nLDL, however, Fig. 1D shown that S1P and LPA did not induce IL-8 production (*vs untreated=13.57±0.29 ng/mL vs. 5.53±0.59 ng/mL, p<0.01; S1P or LPA vs. untreated=5.56±0.34 ng/mL (S1P) or 5.51±0.50 ng/mL (LPA) vs. 5.53±0.59 ng/mL, not significant).
We next tested whether nLDL induces H2O2 generation and also H2O2 generation is dependent on PTX-sensitive G-protein coupled receptor. As shown in Fig 2, incubation of hAoSMCs with nLDL resulted in a significant increase of DCF fluorescence (*vs. untreated=7.42±0.72 vs.1.00±0.14, p<0.01, n=3). On the other hand, preincubation of the cells with PTX (#vs. nLDL=1.10±0.10 vs. 7.42±0.72, p<0.01, n=3) or PEG-Cat (##vs. nLDL=0.92±0.19 vs. 7.42±0.72, p<0.01, n=3) significantly prevented the increase of nLDL-induced DCF fluorescence. Furthermore, H2O2 markedly increased DCF fluorescence (**vs. untreated=5.36±0.38 vs. 1±0.14, p<0.01, n=3). Fig. 2B shows relative fluorescence values as bar graph from 3 independent experiments.
Since p38 MAPK is a critical component of redox-sensitive signaling pathways in VSMCs,17-19 we tested whether nLDL-induced p38 MAPK activation and IL-8 production were associated with increased intracellular H2O2 generation. As shown in Fig. 3A, nLDL-induced p38 MAPK activation was completely blocked with pretreatment of the cells with cell-permeable PEG-Cat. Furthermore, increased IL-8 production by nLDL was also abrogated by incubation with PEG-Cat (Fig. 3B, *vs. untreated=15.12±0.36 ng/mL vs. 4.83±0.29 ng/mL, p<0.01; #vs. nLDL=4.98±0.41 ng/mL vs.15.12±0.36 ng/mL, p<0.01). Interestingly, exogenous H2O2 at a low concentration of 10 µmol/L induced the phosphorylation of p38 MAPK as early as 10 minutes (Fig. 3C) and increased IL-8 production in a dose-dependent manner (Fig. 3D, *vs. untreated control=6.42±0.39 ng/mL vs. 4.95±0.36 ng/mL, p<0.05; #vs. untreated=10.23±1.26 ng/mL vs. 4.95±0.36 ng/mL, p<0.01). Since exogenous H2O2 at concentrations higher than 20 µmol/L significantly decreased cell viability as early as 1 hour, we failed to completely mimic IL-8 production to the level of nLDL stimulation. Therefore, these data suggest that intracellular localization of H2O2 generation could be important in the activation of redox-sensitive protein kinases.
We next examined whether the secreted IL-8 had any effect on the migration of hAoSMCs that is one of critical phenomena of hAoSMC during atherogenesis. Therefore, the effect of IL-8 on hAoSMC migration was determined by Transwell assay as described in the methods section. As shown in Fig. 4, IL-8 treatment at 15 ng/mL significantly increased hAoSMCs migration (*vs. untreated=1.88±0.30 vs. 1.04±0.30, p<0.01). The migration activity of IL-8 was completely blocked with neutralizing antiserum (0.5 µg/mL) against IL-8 (#vs. IL-8=0.98±0.16 vs.1.88±0.30, p<0.01). PDGF-BB as a positive control increased the migration as much as 2.45±0.11 fold.
Herein, we have demonstrated that nLDL stimulation transduces a signal via the PTX-sensitive G-protein coupled receptor and redox-imbalance by generating H2O2, both of which are important signaling events in p38 MAPK activation and IL-8 production in hAoSMCs. Furthermore, IL-8 at a concentration of 15 ng/mL significantly increased hAoSMCs migration that was blocked with IL-8-neutralizing antiserum.
nLDL evoked proinflammation by upregulating IL-8 production via phosphorylation of p38 MAPK and activation of transcription factor, AP-1,16 and also induced proliferation via PTX-sensitive G-protein coupled receptor-dependent protein kinase C (PKC) activation in hAoSMCs.20 Consistent with the effect of nLDL on hAoSMC proliferation, increased IL-8 production was completely blocked by PTX treatment. However, CTX, Gαs-protein coupled receptor inhibitor, heparin (classical LDL receptor inhibitor), and genistein (a tyrosine kinase inhibitor), had no effect on nLDL-induced IL-8 production (Fig. 1). These data suggest that nLDL transfers signals for IL-8 production through the common Gαi/o-protein coupled receptor instead of both the conventional LDL receptor, LDLR, and endothelial differentiation gene (EDG) receptor. The Gαi/o-protein coupled receptor appears to be responsible for activation of Erk1/2 MAPK20 and p38 MAPK during nLDL signal transduction.
Since that atherogenesis is generally viewed as a chronic inflammatory disease, an altered redox-state results in enhancing the expression of proinflammatory genes that facilitate interactions between endothelium and leukocytes. Increased expression of proinflammatory genes is mediated through redox-sensitive transcription factors, such as NF-kB, AP-1, Egr-1, and HIF-1β, which are also involved in growth, vascular remodeling, and atherogenesis of VSMCs. ROS exerts intracellular signaling processes by activating mitogen activated protein kinase (MAPK) and receptor- or non-receptor-tyrosine kinases, although previous studies have revealed contradictory results on the physiological roles of intracellular H2O2 and O2- in VSMCs. VSMCs proliferation induced by O2- generating LY8358321 and nLDL-dependent proliferation is blunted by the administration of PEG-SOD,21,22 suggesting that intracellular O2- plays an important role in VSMCs proliferation. On the other hand, angiotensin II-dependent p38 MAPK activation is a process associated with H2O2 generation19 and activated p38 MAPK stimulates cell migration.23 These results are consistent with our finding that nLDL increased intracellular H2O2 generation, resulting in activation of p38 MAPK associated with IL-8 production (Fig. 3).
Every cellular component in the vascular wall is known to be a potential source of IL-8.16,24,25 Produced IL-8 has some functional characteristics similar to other cytokines. IL-8 is resistant to temperature, proteolysis, and acidic environments, making it as an ideal candidate molecule for acute inflammation.26 Another characteristic of IL-8 is its relative longevity at sites of acute inflammation.26 IL-8 is produced early in the inflammation response, but remains active for a prolonged period of time, even days and weeks. A third interesting aspect of IL-8 is that the expression is highly sensitive to oxidants and anti-oxidants substantially reduce IL-8 gene expression.27 Furthermore, IL-8 contributes to atherogenesis by modulating monocyte-endothelial interaction,13 stimulating VSMCs proliferation,15 inhibiting tissue inhibitor of metallopeptidase (TIMP-1) expression,28 and inducing angiogenic activity.29 On the other hand, the neutralization of IL-8 significantly reduces the degree of necrosis in a rabbit model of myocardial ischemia-reperfusion injury.30 The present study together with all the above-mentioned observations indicate that IL-8 may be either a marker or a potential therapeutic target of atherosclerosis.31
In summary, this study demonstrated that nLDL transferred a signal through PTX-sensitive receptor and increased H2O2 generation that were key phenomena for p38 MAPK activation and IL-8 production in hAoSMCs. Furthermore, IL-8 treatment increased migration activity of hAoSMCs.
Figures and Tables
 | Fig. 1Gαi/o-protein coupled receptor is responsible for IL-8 production in nLDL-stimulated hAoSMCs. (A) nLDL (100 µg/mL) significantly induced IL-8 production (*vs. untreated, p<0.01) that was completely abrogated with preincubation of the cells with PTX (100 ng/mL, **vs. nLDL, p<0.01), but CTX (100 ng/mL), heparin (2 mg/mL), and genistein (100 µmol/L) were not effective on nLDL-induced IL-8 production (#vs. nLDL, not significant). n=8 from 4 experiments. (B) hAoSMCs were incubated with nLDL (100 µg/mL) for 10 minutes in the presence or absence of PTX and CTX. PTX completely inhibited nLDL-induced phosphorylation of p38 MAPK, but CTX, heparin, and genistein had no effect (C). (D) Incubation of agonists (S1P, sphingosine-1-phosphate, 100 nmol/L; LPA, lysophosphatidic acid, 25 µmol/L) that stimulate Gαi/o-protein coupled receptors did not increase IL-8 production (*vs. nLDL, p<0.01; **vs. untreated, not significant, n=4). Representative blot from 3 experiments. Phosphate-buffered saline (PBS) was used as untreated control. nLDL, native low density-lipoprotein; hAoSMCs, human aortic smooth muscle cells; IL-8, interleukin-8; PTX, pertussis toxin; CTX, cholera toxin. |
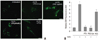 | Fig. 2PTX-sensitive Gαi/o-protein coupled receptor is involves in nLDL-dependent H2O2 generation. (A) DCF-fluorescence images to intracellular H2O2 in hAoSMCs were captured after treatment of nLDL (100 µg/mL) for 30 minutes in the presence or absence of PTX and PEG-Cat (500 U/mL). Treatment of nLDL led to an increase in H2O2 production that was abrogated with preincubation of PTX and PEG-Cat. Exogenous H2O2 (10 µmol/L, 10 minutes) was used as a control. Bar length is 10 µm. (B) Relative intensities of DCF fluorescences were presented as a bar graph (*vs. untreated, p<0.01; #vs. nLDL, p<0.01; ##vs. nLDL, p<0.01; **vs. untreated, p<0.01; n=3). |
 | Fig. 3Effect of nLDL-dependent H2O2 generation on IL-8 production. Preincubation of PEG-Cat (500 U/mL) blocked nLDL-induced phosphorylation of p38 MAPK (A) that was resulted in significant inhibition of IL-8 production (B)(*vs. untreated, p<0.01; #vs. nLDL, p<0.01; n=8 from 4 experiments). Exogenous H2O2 at 10 and 20 µmol/L markedly increased p38 MAPK phosphorylation at 10 minutes (C), resulting in induction of IL-8 production (D)(*vs. untreated, p<0.05; #vs. untreated, p<0.01, n=8 from 4 experiments). |
 | Fig. 4IL-8 stimulates the migration of hAoSMCs. For the migration assay, hAoSMCs suspensions were loaded into the upper wells of Transwell plates, whereas IL-8 (15 ng/mL) alone and IL-8 plus anti-IL-8 antiserum (0.5 µg/mL) were added into the lower wells. After 8 hours, cells that had migrated to the lower side of the filter were stained (A) and counted using an optical microscope at 400X magnification (B). IL-8 increased cell migration (*vs. untreated, p<0.01, n=4), but its neutralizing antibody reversed cell migration back to untreated control levels (#vs. IL-8, p<0.01, n=4). PDGF (PDGF-BB, 20 ng/mL) was used as a positive control. Bar length is 100 µm. PDGF, platelet-derived growth factor. |
ACKNOWLEDGEMENTS
This work was supported by a research grant from the Yonsei University Wonju College of Medicine (YUWCM-2009-20) and by a 2009 Research Grant from Kangwon National University and the Korea Research Foundation Grand founded by the Korea government (MEST/MBMRC) and by the Basic Science Research Program of NRF (2010-0023741).
References
2. Steinberg D, Witztum JL. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990. 264:3047–3052.

3. Vega GL, Grundy SM. Primary hypertriglyceridemia with borderline high cholesterol and elevated apolipoprotein B concentrations. Comparison of gemfibrozil vs lovastatin therapy. JAMA. 1990. 264:2759–2763.

4. Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000. 33:Suppl. S85–S97.
5. Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981. 103:181–190.
6. Stary HC, Blankenhorn DH, Chandler AB, Glogov S, Insull W Jr, Richardson M, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992. 12:120–134.

7. Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989. 9:I19–I32.
8. Rosenfeld ME, Tsukada T, Gown AM, Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987. 7:9–23.

9. Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006. 114:1531–1544.

10. Heo KS, Kim DU, Ryoo S, Nam M, Baek ST, Kim L, et al. PPARgamma activation abolishes LDL-induced proliferation of human aortic smooth muscle cells via SOD-mediated down-regulation of superoxide. Biochem Biophys Res Commun. 2007. 359:1017–1023.

11. Lee HS, Son SM, Kim YK, Hong KW, Kim CD. NAD(P)H oxidase participates in the signaling events in high glucose-induced proliferation of vascular smooth muscle cells. Life Sci. 2003. 72:2719–2730.

12. Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001. 276:21938–21942.

13. Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999. 398:718–723.

14. Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998. 101:353–363.

15. Yue TL, Wang X, Sung CP, Olson B, Mckenna PJ, Gu JL, et al. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994. 75:1–7.

16. Ryoo SW, Kim DU, Won M, Chung KS, Jang YS, Oh GT, et al. Native LDL induces interleukin-8 expression via H2O2, p38 Kinase, and activator protein-1 in human aortic smooth muscle cells. Cardiovasc Res. 2004. 62:185–193.

17. Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003. 108:1746–1752.

18. Maupas-Schwalm F, Augé N, Robinet C, Cambus JP, Parsons SJ, Salvayre R, et al. The sphingomyelin/ceramide pathway is involved in ERK1/2 phosphorylation, cell proliferation, and uPAR overexpression induced by tissue-type plasminogen activator. FASEB J. 2004. 18:1398–1400.

19. Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998. 273:15022–15029.

20. Heo KS, Kim DU, Kim L, Nam M, Baek ST, Park SK, et al. Activation of PKCbeta(II) and PKCtheta is essential for LDL-induced cell proliferation of human aortic smooth muscle cells via Gi-mediated Erk1/2 activation and Egr-1 upregulation. Biochem Biophys Res Commun. 2008. 368:126–131.

21. Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells. Circ Res. 1995. 77:29–36.

22. Locher R, Brandes RP, Vetter W, Barton M. Native LDL induces proliferation of human vascular smooth muscle cells via redox-mediated activation of ERK 1/2 mitogen-activated protein kinases. Hypertension. 2002. 39:645–650.

23. Mugabe BE, Yaghini FA, Song CY, Buharalioglu CK, Waters CM, Malik KU. Angiotensin II-induced migration of vascular smooth muscle cells is mediated by p38 mitogen-activated protein kinase-activated c-Src through spleen tyrosine kinase and epidermal growth factor receptor transactivation. J Pharmacol Exp Ther. 2010. 332:116–124.

24. Ito T, Ikeda U, Yamamoto K, Shimada K. Regulation of interleukin-8 expression by HMG-CoA reductase inhibitors in human vascular smooth muscle cells. Atherosclerosis. 2002. 165:51–55.

25. Geisel J, Jödden V, Obeid R, Knapp JP, Bodis M, Herrmann W. Stimulatory effect of homocysteine on interleukin-8 expression in human endothelial cells. Clin Chem Lab Med. 2003. 41:1045–1048.

26. DeForge LE, Fantone JC, Kenney JS, Remick DG. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992. 90:2123–2129.

27. DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993. 268:25568–25576.

28. Moreau M, Brocheriou I, Petit L, Ninio E, Chapman MJ, Rouis M. Interleukin-8 mediates downregulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation. 1999. 99:420–426.

29. Simonini A, Moscucci M, Muller DW, Bates ER, Pagani FD, Burdick MD, et al. IL-8 is an angiogenic factor in human coronary atherectomy tissue. Circulation. 2000. 101:1519–1526.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download