Abstract
Purpose
Visceral fat (VF) is closely associated with many metabolic risk factors and is also known to be a strong predictive factor for severe metabolic complications in adults. But there are only a few studies concerning the association of VF and risk factors for metabolic syndrome (MS) in children and adolescents. In our study, we emphasized the association of VF [measured by VF computed tomography (VFCT)] and risk factors for metabolic syndrome in children and adolescents.
Materials and Methods
The subjects were outpatients aged 6 to 18 years who underwent VFCT in the family medicine of The Catholic University of Korea from January 2005 to August 2009. There were 82 patients in total (42 children, 40 adolescents). Height, weight, blood pressure (BP), blood tests, body composition analysis and VF were measured. The three groups were also classified by metabolic score.
Results
In children, only high density lipoprotein cholesterol (HDL-C) showed a statistically significant difference, while in adolescents, triglyceride, HDL-C, BP, body mass index (BMI), waist circumference (WC) and VFA showed statistically significant differences. In terms of VFA, fasting glucose, BP, BMI, basal metabolic rate (BMR) and WC showed statistically significant differences. BMI showed a statistically significant difference in terms of BP, BMR, WC, VFA and HDL-C.
Conclusion
There is a need to acknowledge the statistically significant associations of VF and risk factors for MS in children and adolescents. Screening tests for BP, cholesterol, fasting glucose and WC should be given in clinics for children and adolescents so that MS can be detected and its risk factors treated early.
Go to : 
In recent years, the prevalence of overweight or obese children and adolescents has been rapidly increasing.1 The rate of increase exceeds even that of adults.2 A 10-fold increase (1.7→17.9%) for boys and a 4.5-fold increase (2.4→10.9%) for girls was reported in Seoul between 1979 and 2002.3 Obese children and adolescents are more likely to develop into obese adults,4,5 and also have a high risk of affliction with associated metabolic complications as they grow to adults. For instance, Type II diabetes mellitus, hypertension, dyslipidemia and atherosclerosis can develop.6-8 This fact causes excessive personal and social cost, and is the reason why more aggressive intervention and countermeasures must be implemented to control metabolic syndrome (MS) in Korean children and adolescents.9,10 Until now, there has been insufficient information concerning diagnostic criteria for MS in children and adolescents. In children and adolescents, physical as well as physiological changes are taking place. Blood pressure (BP), body mass index (BMI) and blood cholesterol levels vary according to age, which makes it hard to set a cut off value for diagnostic criteria. Fortunately, in 2007, the International Diabetes Federation reported the diagnostic criteria for MS in children and adolescents,11 and this criterion is being used along with the variation for children and adolescents of National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria. VF is closely associated with many metabolic risk factors and is also known to be a strong predictive factor of severe metabolic complications in adults.12-17 However, few studies have been done concerning the association of VF and risk factors for MS in children and adolescents. In our study, we emphasized the association between VF [measured by VF computed tomography (VFCT)] and risk factors for MS in children and adolescents.
Go to : 
All subjects gave written informed consent before taking part in this study. The subjects were outpatients, aged 6 to 18 years, who underwent VFCT in the family medicine clinic of The Catholic University of Korea located in Seoul and Gyeonggi province from January 2005 to August 2009. There were 82 patients in total (42 children, 40 adolescents). Height, weight, BP, blood tests, body composition analysis and VF were measured. The three groups of children and adolescents were then classified by metabolic scores. The study was approved by The Catholic University of Korea's ethics committee.
Height and weight were measured with the patient wearing light clothes or only a gown. We measured up to 0.1 kg, 0.1 cm respectively. Waist circumference (WC) was measured while the patient exhaled slightly, with no pressure applied, and then we measured the area between the lowest rib and the upper part of the iliac crest. BP was measured according to the recommendations from the American Heart Association (AHA). The patients relaxed for more than 10 minutes and while they were sitting we measured twice, each measurement 5 minutes apart. The final BP was the mean value of these two measurements. The patients did not eat anything for 12 hours prior to the blood tests. After withdrawing 15 mL of blood, it was sent to the laboratory for fasting glucose, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C) etc. Body composition analysis was made using Biospace Corp. In Body 4.0 body fat analyzer. VFCT was referred to the radiologist. The lower abdomen of each subject was covered with lead protectors in order to protect them from radioactive exposure. VFCT was done around the 4th lumbar vertebra area where subcutaneous and visceral fat were measured.
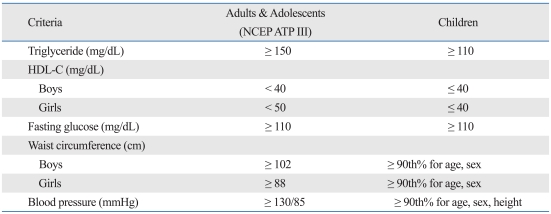
According to NCEP ATP III criteria, the MS score was classified as 0-1, 2-3 and 4-5. We compared fasting blood glucose, total blood cholesterol, blood triglyceride, blood HDL-C, systolic and diastolic BP, basal metabolic rate (BMR), WC, subcutaneous fat, VF and total fat area. All results were denoted as a mean ± S.D. A t-test for independent samples was used to assess the differences between two groups. The three groups were classified by MS score and analyzed by an ANOVA test. Visceral fat area (VFA), BMI and other risk factors were analyzed by the Pearson correlation. Statistical analysis of the data was done by Statistical Package for Social Science (SPSS) 15.0 for Windows (SPSS Inc, Chicago, IL, USA). We considered a p value of less than 0.05 to be statistically significant.
Go to : 
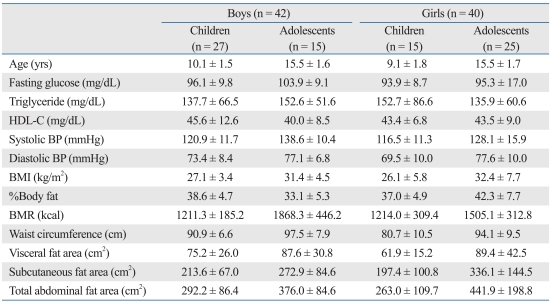
There were 82 subjects in total (children: 42, adolescents: 40). The mean age of the children was 10.1 ± 1.5 yrs for boys and 9.1 ± 1.8 yrs for girls. The mean age of the adolescents was 15.5 ± 1.6 yrs for boys and 15.5 ± 1.7 yrs for girls. Fasting blood glucose (FBG) was 96.1 ± 9.8 mg/dL (boys) and 93.9 ± 8.7 mg/dL (girls) in children, and 103.9 ± 9.1 mg/dL (boys) and 95.3 ± 17.0 mg/dL (girls) in adolescents. Blood triglyceride levels were 137.7 ± 66.5 mg/dL (boys) and 152.7 ± 86.6 mg/dL (girls) in children, and 152.6 ± 51.6 mg/dL (boys) and 135.9 ± 60.6 mg/dL (girls) in adolescents. Blood HDL-C was 45.6 ± 12.6 mg/dL (boys) and 43.4 ± 6.8 mg/dL (girls) in children, and 40.0 ± 8.5 mg/dL (boys) and 43.5 ± 9.0 mg/dL (girls) in adolescents. Systolic BP was 120.9 ± 11.7 mmHg (boys) and 116.5 ± 11.3 mmHg (girls) in children, and 138.6 ± 10.4 mmHg (boys) and 128.1 ± 15.9 mmHg (girls) in adolescents. BMR was 1211.3 ± 185.2 kcal (boys) and 1214.0 ± 309.4 kcal (girls) in children, and 1868.3 ± 446.2 kcal (boys) and 1505.1 ± 312.8 kcal (girls) in adolescents. WC was 90.9 ± 6.6 cm (boys) and 80.7 ± 10.5 cm (girls) in children, and 97.5 ± 7.9 cm (boys) and 94.1 ± 9.5 cm (girls) in adolescents. VFA was 75.2 ± 26.0 cm2 (boys) and 61.9 ± 15.2 cm2 (girls) in children, and 87.6 ± 30.3 cm2 (boys) and 89.4 ± 42.5 cm2 (girls) in adolescents. BMI was 27.1 ± 3.4 (kg/m2) (boys) and 26.1 ± 5.8 (kg/m2) (girls) in children, and 31.4 ± 4.5 (kg/m2) (boys) and 32.4 ± 7.7 (kg/m2) (girls) in adolescents. Percentage body fat (%BF) was 38.6 ± 4.7% (boys) and 37.0 ± 4.9% (girls) in children, and 33.1 ± 5.3% (boys) and 42.3 ± 7.7% (girls) in adolescents. Subcutaneous fat area (SCFA) was 213.6 ± 67.0 cm2 (boys) and 197.4 ± 100.8 cm2 (girls) in children, and 272.9 ± 84.6 cm2 (boys) and 336.1 ± 144.5 cm2 (girls) in adolescents. The total abdominal fat area (TAFA) was 292.2 ± 86.4 cm2 (boys) and 263.0 ± 109.7 cm2 (girls) in children, and 376.0 ± 84.6 cm2 (boys) and 441.9 ± 198.8 cm2 (girls) in adolescents (Table 2).
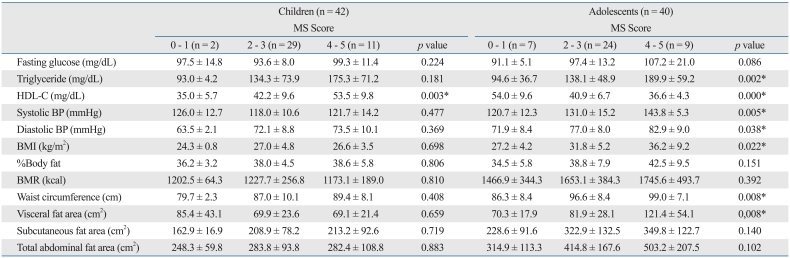
In our study we classified the 82 subjects according to the NCEP ATP III criteria and the scores for MS were classified using 0-1, 2-3 and 4-5.
1) FBG, %BF, BMR, SCFA and TAFA: these factors did not show statistically significant differences between each group in children and adolescents (p < 0.05).
2) Blood triglyceride, systolic and diastolic BP, BMI, WC and VFA: these factors did not show statistically significant differences between groups in children, but did show statistically significant differences in adolescents (p < 0.05).
3) Blood HDL-C: this factor showed statistically significant differences between groups in both children and adolescents.
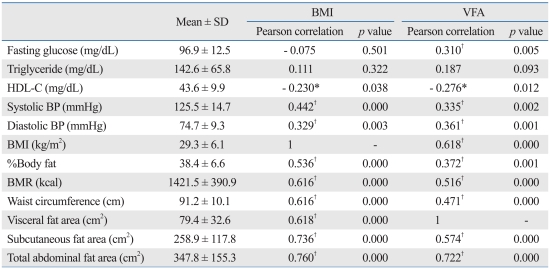
1) BMI: At a p value of 0.01, there were statistically significant differences in systolic and diastolic BP, %BF, BMR, WC, VFA, SCFA and TAFA. At a p value of 0.05, there were statistically significant differences in HDL-C.
2) VFA: At the p value of 0.01 there were a statistically significant differences in fasting glucose, systolic and diastolic BP, BMI, %BF, BMR, WC, SCFA and TAFA. At the p value of 0.05, there were statistically significant differences in HDL-C.
Go to : 
It is known that obesity in children and adolescents is related to high prevalence of metabolic complications.19-21 In 2004 Chang, et al.22 reported that the prevalence of MS in children and adolescents was 37.5% (boys: 38.7%, girls: 35.2%) by the corrected criteria of NCEP ATP III. Considering that the prevalence of obesity in children and adolescents in the United States is 28.7%, we believe the time, method and criteria of the study is critical in Korea.23,24
There have not been many studies concerning the relationship between VF and MS in children and adolescents. In 2008, Kim and Park25 reported in one study that there is a relationship between abdominal fat and cardiovascular risk factors and metabolic risk factors in obese Korean children and adolescents. VF was found to be independently associated with BP, blood triglyceride, blood HDL-C, FBG and homeostasis model assessment (HOMA) score. Also, Druet, et al.26 reported that VF was independently related to BP, blood triglyceride and blood HDL-C in obese children and adolescents. Thus, VF has been identified as a severe risk factor for MS.
In our study of 82 subjects, we first classified them as children or adolescents and then classified the risk factor scores in groups 0-1, 2-3 and 4-5 according to NCEP ATP III criteria. In children, only HDL-C showed statistically significant differences between three groups, while in adolescents, blood triglyceride, HDL-C, systolic and diastolic BP, BMI, WC and VFA all showed statistically significant differences. MS score elevation in children and adolescents indicates significant increases in the risk factors described above. The severity of MS is related to metabolic complications, including cardiovascular complications. Our study had the same results as other studies. In Korean studies, the risk of cardiovascular disease was higher when more than one metabolic risk factor was involved.27 In our study, we compared the associations between VF (measured by VFCT) and BMI between risk factors for MS by groups. VFA was statistically significant in relation to fasting glucose, systolic and diastolic BP, BMI, %BF, BMR, WC, SCFA, TAFA and HDL-C, while BMI was related to systolic and diastolic BP, %BF, BMR, WC, VFA, SCFA, TAFA and HDL-C. The aforementioned associations between VF and risk factors for MS suggest the need for abdominal fat measurement.28 But there are some groups who oppose VFCT due to radioactive exposure.
Our study had two important limitations. First of all, diagnosis of MS in children and adolescents is not standardized, because children and adolescents continue to grow, making it difficult to set a normal value and to have long-term follow-up. Also, there is little interest in MS in children and adolescents worldwide, including in the Republic of Korea. Second, we enrolled only a total of 82 subjects because it was difficult to find subjects due to fear of radioactive exposure from VFCT. Safety and verification of radioactive exposure should be studied.
According to AHA, as the prevalence of MS increases in children and adolescents, severe metabolic complications also increase. In clinics for children and adolescents, screening tests for BP, serum cholesterol, serum glucose and abdominal circumference should be undertaken. Thus, early detection and follow-up of risk factors for MS can be possible. There is a strong need to set a cut-off value for diagnostic criteria of MS risk factors in children and adolescents.
Go to : 
References
1. Lobstein T, Frelut ML. Prevalence of overweight among children in Europe. Obes Rev. 2003; 4:195–200. PMID: 14649370.

2. Popkin BM, Conde W, Hou N, Monteiro C. Is there a lag globally in overweight trends for children compared with adults? Obesity (Silver Spring). 2006; 14:1846–1853. PMID: 17062816.

3. Park YS, Lee DH, Choi JM, Kang YJ, Kim CH. Trend of obesity in school age children in Seoul over the past 23 years. Korean J Pediatr. 2004; 47:247–257.
4. Epstein LH, Wing RR, Valoski A. Childhood obesity. Pediatr Clin North Am. 1985; 32:363–379. PMID: 3887305.

5. Dietz WH Jr. Childhood obesity: susceptibility, cause, and management. J Pediatr. 1983; 103:676–686. PMID: 6631594.

6. Bao W, Srinivasan SR, Valdez R, Greenlund KJ, Wattigney WA, Berenson GS. Longitudinal changes in cardiovascular risk from childhood to young adulthood in offspring of parents with coronary artery disease: the Bogalusa Heart Study. JAMA. 1997; 278:1749–1754. PMID: 9388151.

7. Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999; 83:25F–29F.

9. Seo MJ, Seong JW, Seon KJ, Go BJ, Han JH, Kim SM. Prevalence of the metabolic syndrome in Korean children and adolescents: Korea National Health and Nutrition Survey 2001. J Korean Acad Fam Med. 2006; 27:798–806.
11. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007; 369:2059–2061. PMID: 17586288.

12. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004; 350:2362–2374. PMID: 15175438.

13. Guo SS, Roche AF, Chumlea WC, Gardner JD, Siervogel RM. The predictive value of childhood body mass index values for overweight at age 35 y. Am J Clin Nutr. 1994; 59:810–819. PMID: 8147324.

14. Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004; 89:108–113. PMID: 14715836.

15. Druet C, Dabbas M, Baltakse V, Payen C, Jouret B, Baud C, et al. Insulin resistance and the metabolic syndrome in obese French children. Clin Endocrinol (Oxf). 2006; 64:672–678. PMID: 16712670.

16. Pouliot MC, Després JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992; 41:826–834. PMID: 1612197.

17. Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994; 73:460–468. PMID: 8141087.

18. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003; 157:821–827. PMID: 12912790.
19. Yom HW, Shin JS, Lee HJ, Park SE, Jo SJ, Seo JW. The metabolic syndrome in obese children. Korean J Pediatr Gastroenterol Nutr. 2004; 7:228–238.

20. Cho KY, Park H, Seo JW. The relationship between lifestyle and metabolic syndrome in obese children and adolescents. Korean J Pediatr Gastroenterol Nutr. 2008; 11:150–159.

21. Cho YG, Song HJ, Kang JH. Prevalence of the metabolic syndrome in Korean children and adolescents according to the international diabetes federation definition in children and adolescents. Korean J Fam Med. 2009; 30:261–268.

22. Chang JH, Kim DH, Kim HS, Choi IK, Cheong MY, Kim DK. Prevalence of metabolic syndrome in obese children. Korean J Pediatr. 2004; 47:1149–1156.
23. Stark O, Atkins E, Wolff OH, Douglas JW. Longitudinal study of obesity in the National Survey of Health and Development. Br Med J (Clin Res Ed). 1981; 283:13–17.

24. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004; 350:2362–2374. PMID: 15175438.

25. Kim JA, Park HS. Association of abdominal fat distribution and cardiometabolic risk factors among obese Korean adolescents. Diabetes Metab. 2008; 34:126–130. PMID: 18289908.

26. Druet C, Baltakse V, Chevenne D, Dorgeret S, Zaccaria I, Wang Y, et al. Independent effect of visceral adipose tissue on metabolic syndrome in obese adolescents. Horm Res. 2008; 70:22–28. PMID: 18493146.

27. Park HS, Kim YS, Park SW, Park SJ. Clustering of cardiovascular risk factors and cornary artery disease. J Korean Acad Fam Med. 1998; 19:881–893.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download