Acquired carbapenemases represent a major threat to the clinical utility of all β-lactam antibiotics. Particularly, the emergence of acquired metallo-β-lactamase (MBL) among major gram-negative pathogens [
Pseudomonas aeruginosa (PA),
Acinetobacter spp.,
Enterobacteriaceae] is a matter of particular concern on account of their rapid spread and increasing diversity/number of species involved.
1 However, limited clinical data are available regarding to the clinical significance of infections caused by these strains. Several studies have demonstrated that MBL producer (MP) acquisition is associated with increased mortality in PA infection.
2-
5 However, carbapenem susceptible strains are included in MBL non-producer (MNP) in most studies, thus revealing higher mortality in the MBL producing group. It is well known that acquisition of carbapenem resistance is associated with increased mortality in PA and
Acinetobacter baumannii (AB) infections.
6,
7 Therefore, to characterize the MBL production itself rather than carbapenem resistance, we compared MBL producing PA/AB and imipenem-nonsusceptible MNPs (INMNP) isolated from respiratory and urinary tracts which are common sites of clinical infections and sources of nosocomial spread.
The microbiology laboratory database was reviewed, and we selected imipenem-nonsusceptible PA and AB which had been isolated from sputum or urine for the purpose of microbiologic diagnosis irrespective of hospitalization between January 2007 and June 2007 in a single 2,000-bed tertiary hospital in Seoul, Republic of Korea. Clinical data for the source patients of the isolates were collected by a retrospective analysis of the electronic medical records, and patients were excluded from the analysis if medical records prior to the isolation were not sufficient (e.g., transfer from another hospital). Patients without available follow-up data after discharge were also excluded from the analysis. Isolates referred from outpatient departments (OPD) were included if sufficient medical history was available. All the isolates that had been related with both clinical infections or colonization of the respiratory or urinary tracts were considered eligible for the study. Only the first isolate from each patient was included in the analysis.
The PA/AB strains were identified using conventional techniques and/or ATB 32 GN system (BioMérieux, Marcy-l'Étoile, France).
8 The antimicrobial susceptibility was determined using a disk-diffusion method. Results were interpreted using the guidelines of the Clinical and Laboratory Standards Institute.
9
Among the imipenem-nonsusceptible isolates, carbapenemase production was screened by the imipenem disk Hodge (cloverleaf) test, using MacConkey agar instead of the previously used Mueller-Hinton agar.
10 MBL production was screened by the double-disk synergy test using an imipenem disk and an EDTA (750 µg) plus sodium mercaptoacetic acid (SMA, 2 mg) disk on Mueller-Hinton agar with 10 mm distance from the edge to the edge of the disk.
10 Commercial imipenem disks and media (Becton-Dickinson, Sparks, MD, USA) were used for these tests, while the EDTA-SMA disks were prepared from commercially available chemicals (Sigma Chemical, St. Louis, MO, USA). The bla
IMP-1, bla
VIM-2 and bla
SIM-1 alleles were detected by polymerase chain reaction (PCR) among the screening positive isolates as described previously
11 and the primers used were: IMP1-F 5'-CAT GGT TTG GTG GTT CTT GT-3', IMP1-R 5'-ATA ATT TGG CGG ACT TTG GC-3', VIM2-F 5'-ATG TTC AAA CTT TTG AGT AAG-3', VIM2-R 5'-CTA CTC AAC GAC TGA GCG-3', SIM1-F 5'-TAC AAG GGA TTC GGC ATC G-3' and SIM1-R 5'-TAA TGG CCT GTT CCC ATG TG-3'.
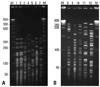
To perform the pulsed field gel electrophoresis (PFGE), genomic DNA of MBL-producing PA and AB was digested with XbaI and SmaI, respectively, as suggested by the manufacturer. Fragments were separated for 20 hours at 6 V/cm at 11℃ using a CHEF-DR II system (Bio-Rad, Hercules, CA, USA). ProMega-Markers Lambda Ladders G3011 (Promega, WI, USA) was used for molecular mass marker.
To identify risk factors contributing to MBL producer acquisition, we reviewed age, gender, Charlson index, APACHE II score, underlying illness, the entire hospital stay period including multiple admissions within six months prior to isolation, intensive care unit (ICU) stay, ventilator care, and antibiotics exposure prior to isolation such as piperacillin/tazobactam, cefoperazone/sulbactam, aztreonam, fluoroquinolones, aminoglycosides, glycopeptides, ceftazidime, and carbapenems. ICU stay, ventilator care and antibiotic exposure were included when each factor lasted more than 48 hours within 28 days prior to isolation. Neutropenia (absolute neutrophil count < 1,000 mm3), immunosuppression (administration of cytostatic agent or glucocorticoid equivalent or more potent than methylprednisolone 20 mg per day for more than 14 days), and antineoplastic treatment within 3 months prior to the isolation were also investigated. To assess the outcome, 28-day case-fatality after isolation and in hospital case-fatality were analyzed. Statistical analyses were performed by SAS version 9.1.3 (SAS Institute, Inc., Cary, NC, USA). All tests were two-tailed, and a p value < 0.05 was considered significant.
During the study period, 432 PA (340 from sputum and 92 from urine) and 199 AB (159 from sputum and 40 from urine) non-repetitive isolates were identified. Among them, 187 (29.6%) were imipenem non-susceptible (130 PA and 57 AB). Eleven isolates were excluded from the analysis because of inadequate availability of their medical records. Finally, 176 isolates consisting of 123 PA (102 from sputum and 21 from urine) and 53 AB (48 from sputum and 5 from urine) were analyzed. The proportion of sources (sputum and urine) was not different between PA and AB (p = 0.19, data not shown).
MBL production was identified in 12 isolates by PCR (6.8%)(
Table 1). The bla
VIM-2 was the most common MBL type (75%, 6 of 7 PA and 3 of 5 AB); two bla
IMP-1 and one bla
SIM-1 sequences were identified in MPs. The proportions of susceptible strains among MPs were 41.7% for piperacillin/tazobactam, fluoroquinolones, and aminoglycosides while few isolates proved to be susceptible to aztreonam (16.7%) and ceftazidime (8.3%). In PFGE analysis, 5 AB isolates revealed different patterns (
Fig. 1). However, 2 isolates among the 7 MBL-producing PA revealed identical PFGE patterns. They were obtained 14 days apart from an inpatient of a rehabilitation center and an outpatient who was regularly visiting OPD of the same center. This last patient was the only one whose culture study was referred from OPD among the 176 imipenem nonsusceptible PA and AB isolates in our study. The proportion of MPs among imipenem non-susceptible isolates was not different according to the species (PA 5.7%/AB 9.4%). However, urine had higher proportion of MP among imipenem non-susceptible isolates than sputum (26.9% vs. 3.3%,
p < 0.001).
With regard to the risk factors associated with MP acquisition, we compared baseline characteristics, underlying morbidity and use of antibiotics within 28 days prior to isolation between MPs and INMNPs (
Table 2). ICU stay (70.1% vs. 25%,
p = 0.003) and mechanical ventilator care prior to PA/AB isolation (56.6% vs. 25%,
p = 0.034) were more frequent in the INMNP group. Exposure to antibiotics was also more frequent in the INMNP group (76.2% vs. 41.7%,
p = 0.015). In the analysis of individual antibiotics, exposure to piperacillin/tazobactam was significantly more frequent in the INMNP group. Therefore, it was not possible to identify significant risk factor related with MP acquisition among imipenem nonsusceptible isolate acquired patients. Twenty-eight-day and in-hospital case-fatalities after imipenem nonsusceptible isolates acquisition revealed no difference between MPs and INMNPs.
With regard to the susceptibilities to the six antibiotics which were frequently used for treatment of PA and AB other than carbapenem (
Table 3), the proportions of susceptible isolates were higher in the MP group for more than half of the analyzed drugs, and those of piperacillin/tazobactam and fluoroquinolones showed statistically significant differences. Regarding to ceftazidime, only one strain (8.3%) was susceptible among MPs, and this proportion was significantly lower than that of INMNPs (61.7%), which is comparable with previous report.
12 However, the aztreonam susceptibility which is known to be uniquely spared among β-lactams against MBL was preserved in only 2 isolates (16.7%) among MPs, and it was not more frequent than that of INMNPs (23.2%).
As mentioned above, the clinical data concerning risk factors and treatment outcomes of infections caused by MBL-producing strains are sparse. Analysis of the antimicrobial chemotherapy administrated before isolation of MPs often revealed that many patients had not been administered with carbapenems, but fluoroquinolones
3,
13 and β-lactam antibiotics.
3,
14,
15 Other reports suggested carbapenem use, ICU stay > 20 days,
15 underlying neurologic disorder, urinary tract infection, renal failure,
3 and neutropenia
14 as contributing factors. However, all of the above mentioned studies compared an MP group with an MNP group irrespective of the susceptibility to carbapenems, which are now considered as a key drug for the treatment of gram-negative bacilli due to the apparently high barrier to resistance acquisition. Therefore, we chose to compare an MP group with an INMNP group rather than with a mere MNP group that includes carbapenem susceptible isolates. Although we failed to identify any significant risk factor, this result might be related to the limited number of MPs in our study population. Further investigation is required to explain this aspect.
We could not investigate the MP acquisition-related mortality because our cases included not only clinical infections, but also colonization. Some authors
2,
4,
5 have reported higher mortality in an MP group. However, they compared the group with MNPs irrespective of carbapenem sensitivity. Although Laupland, et al.
16 observed higher mortality in MBL-producing PA infected patients than in the MBL-nonproducing carbapenem resistant group, more data are required to ascertain the impact of MBL acquisiton on mortality. In a recent study, for example, carbapenem resistance, advanced age, and severity of underlying disease were suggested as independent risk factors for mortality, but not VIM-1 type MBL production among
Klebsiella pneumoniae bloodstream infection patients.
17
As regards to the susceptibility of antibiotics other than imipenem, aztreonam is generally an exception, while MBL production results in high-level resistance to most β-lactam antibiotics. However, equivalent resistance profiles for aztreonam have been observed between the MP group and the MNP group in previous reports.
3,
18,
19 Our study also revealed higher percentage of resistance to aztreonam in the MP group. Resistance to aztreonam in MPs might be explained by extended spectrum β-lactamases (ESBL),
20,
21 AmpC β-lactamases,
22-
24 or other non-enzymatic mechanisms
23 as described before. Therefore, aztreonam may not be an exceptionally useful β-lactam antibiotic for the treatment of MPs in current clinical settings.
The portion of strains showing susceptibility for piperacillin/tazobactam and fluoroquinolones were higher among MPs, and overall prior antibiotics exposure was more frequent in the INMNP group. These findings are not consistent with previous reports,
3,
16,
18 and the discrepancies might be related to our study group which included not only patients with clinical infections but also patients with colonization.
In PFGE analysis, two isolates which were obtained 14 days apart revealed identical PFGE patterns in 7 MBL producing PA. A direct contact between the two patients was not identified. However, one source of major concern is the ability of MBLs to spread rapidly which has been observed in various clinical circumstances.
2,
25,
26 The above cases could be an example of the potential clonal spread of MPs, although the majority of MPs occurred sporadically in our institution, and contribution of MBL to carbapenem resistance was limited, similar to other study performed in the same country.
27
In conclusion, the contribution of MBL to imipenem resistance in PA/AB isolated from sputum and urine was relatively limited in our institution, and there was no identifiable risk factor associated with MPs acquisition, compared with INMNPs. However, the limited susceptibility to aztreonam in the MP group implies that MBL producing strains commonly carry other resistance mechanisms such as ESBLs, AmpC β-lactamases, and other non-enzymatic mechanisms.
Our study has several limitations. First, the number of MPs used for the study was small, and that could have impaired our ability to identify significant risk factors associated with acquisition of MPs. Subgroup analysis according to isolation sources was also not feasible because of the small size of the population under investigation. Second, we included all isolates from the respiratory and urinary tract, therefore, our results might not be generalized as overt clinical infections. Further analysis of a significant number of infection cases is required to more accurately assess the clinical impact of MPs.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download