Abstract
Purpose
In some patients with an implantable cardioverter defibrillator (ICD), multiple episodes of electrical storm (ES) can occur. We assessed the prevalence, features, and predictors of ES in patients with ICD.
Materials and Methods
Eighty-five patients with an ICD were analyzed. ES was defined as the occurrence of two or more ventricular tachyarrhythmias within 24 hours.
Results
Twenty-six patients experienced at least one ES episode, and 16 patients experienced two or more ES episodes. The first ES occurred 209 ± 277 days after ICD implantation. In most ES cases, the index arrhythmia was ventricular tachycardia (65%). There were no obvious etiologic factors at the onset of most ES episodes (57%). More patients with a structurally normal heart (p = 0.043) or ventricular fibrillation (VF) as the index arrhythmia (p = 0.017) were in the ES-free group. Kaplan-Meier estimates and a log-rank test showed that patients with nonischemic dilated cardiomyopathy (DCMP) (log-rank test, p = 0.016) or with left ventricular ejection fraction < 35% (p = 0.032) were more likely to experience ES, and that patients with VF (p = 0.047) were less affected by ES. Cox proportional hazard regression analysis showed that nonischemic DCMP correlated with a greater probability of ES (hazard ratio, 3.71; 95% confidence interval, 1.16-11.85; p = 0.027).
Sudden cardiac death remains one of the leading causes of death.1 An implantable cardioverter defibrillator (ICD) prolongs the lifespan of patients when used for primary or secondary prophylaxis of sudden cardiac death.2-4 Patients with an ICD can develop a so-called electrical storm (ES) during follow-up.5-8 Several studies have assessed the prevalence and possible predictors of ES in patients with an ICD,5,8-13 but there are insufficient data about ES in patients with an ICD and especially about the predictors of ES.5,10 We examined the incidence, features, and timing of the occurrence and predictors of ES in patients with an ICD.
The medical records of 85 patients with an ICD implanted from October 1999 to April 2009, who were treated at Samsung Medical Center in Seoul, Korea, were analyzed retrospectively. All patients gave written informed consent for the procedure of ICD implantation. Of these patients, any patient who had had one or more episode of ES was included in the study population. All arrhythmias detected during an ES episode were also reviewed. The study was approved by the regional committee for ethnics in medical research.
The indication for ICD implantation was defined as secondary prevention in patients who had experienced aborted sudden cardiac death, sustained ventricular tachyarrhythmia, or in the context of presumed tachyarrhythmic syncopal attacks.14 The indication for ICD implantation in all other patients was categorized as primary prevention.
The ICDs were manufactured by St. Jude Medical, Inc. (n = 45), Medtronic, Inc. (n = 38), or Guidant Corp. (n = 2). Single chamber devices were implanted in 77 patients, and double chamber devices in 8 patients. A total of 94 procedures were performed in these 85 patients.
Ventricular tachyarrhythmia episodes were appropriately detected by the device. Some devices recorded templates of the ventricular electrogram during sinus rhythm. During a tachyarrhythmia, the device compared the electrograms during the tachycardia to the baseline. Mean detection cutoff for slow ventricular tachycardia (VT) was 380 milliseconds (159 beats/min), and 332 milliseconds (181 beats/min) for fast VT, and 297 milliseconds (202 beats/min) for ventricular fibrillation (VF). Also, 5 patients were diagnosed to have VT, based on surface electrocardiogram during hospitalization.
We often attempted to tailor therapies based upon the results of electrophysiologic study and/or a patient's arrhythmia history (patient-specific tailored programming). ICDs could be programmed to provide different therapies to tachyarrhythmias in up to three heart rate zones. In each therapy zone, bursts of antitachycardia pacing (APT), cardioversion, or defibrillation could be delivered. Although a variety of algorithms exist, APT was usually programmed to be delivered at a rate that is slightly faster (at a cycle length 10 to 12 percent shorter) than the rate of the detected tachycardia. Devices were often programmed to deliver synchronized cardioversion for tachyarrhythmias in this range (heart rate below 160 or 180 beats/min), and usually programmed to deliver unsynchronized shocks for very rapid ventricular arrhythmias (heart rate > 180 or 200 beats/min).
After ICD implantation, the patients were followed up in our outpatient ICD clinic. The devices were interrogated, and the complete set of data (including intracardiac electrograms) was recorded. Blood samples, echocardiography, coronary angiography, reprogramming of ICD, or adjustment of drug therapy were performed as needed according to the events recorded and factors deemed causative.
For this study, we defined ES as the occurrence of two or more ventricular tachyarrhythmia within 24 hours, which need immediate electrical therapy (ATP and/or shock) to be terminated, separated by a period of sinus rhythm.9,15-17 We divided patients into two groups according to occurrence of ES. Individual demographic and clinical data were entered into a continually updated database. Multiple device activation from supraventricular tachyarrhythmias or other factors not related to ventricular tachyarrhythmic events (defined as "inappropriate therapy"), as judged by the clinical picture and analysis of stored electrograms, were excluded.
The data are expressed as mean ± standard deviation or as percentages. Differences in the frequency of characteristics were assessed by the two independent-samples tests for continuous variables. Chi-square statistics (or Fisher's exact test if applicable) were used to analyze discrete variables. We used survival analysis method to investigate the relationship between the significance of clinical outcomes and ES. Cumulative incidence of clinical outcomes was estimated by the Kaplan-Meier method, and differences were assessed with the log-rank test for ES. We used Cox proportional hazard regression analysis with the forward selection method to identify precipitating factors. All tests of statistical significance were two-tailed, and a p value of 0.05 was considered significant. Statistical analyses were performed using SPSS statistical software (version 15.0; SPSS Inc., Chicago, IL, USA).
Eighty-five patients with an ICD were followed for a mean of 861 ± 715 days. We compared the baseline characteristics between patients with ES (n = 26, 31%) and without ES (n = 59, 69%) during follow-up. The mean follow-up period was 990 ± 742 days for the patients with ES, and 804 ± 702 days for those without ES. The clinical characteristics in both groups were similar, except for two clinical characteristics: a structurally normal heart and VF as the index arrhythmia for ICD implantation, which were more frequent in the ES-free patients (Table 1). Twenty-six patients experienced at least one ES episode. Overall, 115 ES episodes were recorded (median, 2 ES per patient; range, 1-45), and 16 (62%) patients in the ES group experienced two or more ES episodes. ES occurred first at a mean of 209 ± 277 days after ICD implantation, and the mean duration between the first and second ES occurrence was 251 ± 326 days. Shocks alone were used to treat 28 of the 115 initial ES episodes (n = 28, 24%), and the remaining 87 episodes were treated with ATP alone (n = 55, 48%) or a combination of shocks and ATP (n = 32, 28%). In most ES cases, the recorded index arrhythmia was ventricular tachycardia (VT, 65%). VF and both VT and VF occurred in 11% and 24% of the ES episodes, respectively. One patient experienced a storm immediately following ICD implantation (on the same day). Details of the clinical characteristics and management of the patients with ES are presented in Table 2. Three patients have had heart transplants; two in the ES group and one in the ES-free group. Nine deaths occurred, five in the ES group and four in the ES-free group.
No obvious etiologic factor was evident at the onset of the storm in 65 (57%) ES episodes. Worsening congestive heart failure was evident as an etiologic factor in 19 (17%) cases. Fifteen (13%) storms occurred in the context of other medical illnesses. Six (5%) ES episodes were associated with either non-compliance or adjustment of antiarrhythmics or other medications. Three (3%) ES episodes occurred during and around periods of excess alcohol consumption, and four (3%) storms followed periods of unusual physical or emotional stress. One ES episode was probably caused by hyperkalemia (5.9 mmol/L) and one ES episode was caused by malposition of an ICD lead (Fig. 1).
Chi-square tests showed that more patients with a structurally normal heart (p = 0.043) or VF as the index arrhythmia for ICD (p = 0.017) were in the ES-free group. The odds ratios of a structurally normal heart and VF as the index arrhythmia for ICD were 0.285 [95% confidence interval (CI): 0.087-0.933] and 0.273 (95% CI: 0.091-0.824), respectively. These data suggest that having VF as the index arrhythmia or a structurally normal heart seemed to protect against ES. We intended to find predictive factors for ES. The Kaplan-Meier estimate and log-rank test showed that patients with nonischemic DCMP as the underlying heart disease (log-rank test, p = 0.016) or with left ventricular ejection fraction (LVEF) < 35% (log-rank test, p = 0.032) were more likely to experience ES, and that patients with VF as the index arrhythmia (log-rank test, p = 0.047) were less affected by ES (Table 3). The Cox proportional hazard regression analysis showed that only nonischemic DCMP as the underlying heart disease (hazard ratio, 3.71; 95% CI: 1.16-11.85; p = 0.027) was significantly and independently associated with a greater probability of ES during follow-up.
Our study reveals several findings about the occurrence of ES in ICD patients. Twenty-six (31%) patients who underwent ICD placement experienced at least one ES episode, and a substantial proportion (62%) of patients experienced repeated ES episodes. Most ES episodes involved VT and occurred usually without obvious etiologic factors (57%). Our data suggest that patients with nonischemic DCMP as the underlying heart disease are at greater risk for ES. The finding that patients with VF as the index ventricular tachyarrhythmia or with a structurally normal heart were at lower risk of ES is interesting.
Several studies have attempted to identify the risk factors for the development of ES. Verma, et al.9 demonstrated that coronary artery disease as the underlying heart disease was an independent predictor of ES in 2,028 patients with ICDs. However, the Cox proportion hazard regression analysis of our data indicated that nonischemic DCMP as the underlying heart disease was an independent predictor of ES. The incidence of ES in our study is similar to 10-28% range reported during follow-up durations of 13-33 months.5,10,18,19 Our study and previous reports together suggest that most storm episodes (86-97%) are caused by monomorphic VT. VF alone accounts for 1-21% of ES, mixed VT/VF for 3-14%, and polymorphic VT 2-8%.5,18,20-22 Our findings are consistent with these values. In our study, most of the index arrhythmias causing ES were VT (65%). Identifiable triggers such as aggravation of congestive heart failure (31%) or electrolyte disorders (20%) have been reported.23 Most (57%) of the ES episodes in our study had no obvious etiologic factors, although we found other identifiable precipitating factors, such as worsening congestive heart failure (17%), acute medical illness (13%), and physical or emotional stress (3%).
Chi-square analysis with odds ratios, the Kaplan-Meier estimate, and the log-rank test identified two protective factors against ES: VF as the presenting arrhythmia and a structurally normal heart. Consistent with our trial, Exner, et al.10 reported that the majority of initial ES episodes were related to episodes of VT (77 of 90 initial episodes; 86%), and the residual 14% of episodes were attributed to VF or a mixture of VT and VF. Raitt, et al.24 also showed that patients who were included in study after an episode of VT, were more likely to experience an episode of VT (73.5% vs. 30.1%, p < 0.001), and were less likely to have an episode of VF (18.3% vs. 28.0%, p = 0.013) than those who enrolled after an episode of VF in the AVID trial, which suggested that there are significant differences in the electrophysiologic features of these 2 groups. On the other hand, Verma, et al.9 described that 208 (10%) patients presented with ES in 2,028 patients who were evaluated in the ICD clinic, and VF was the reason of ES in 99 of 208 patients, for an overall incidence of 48%.
Our study is limited by the relatively small sample size comprising a selected patient population and the retrospective, observational single-center design. Our results should be confirmed in a large, prospective study.
ES is a relatively frequent complication that may occur at any time after ICD implantation and can become a recurrent event in ICD recipients. Our data provide evidence to indicate that nonischemic DCMP as the underlying heart disease is associated with an increased risk of ES, and that patients with VF as the index arrhythmia or a structurally normal heart are less likely to experience ES.
Figures and Tables
Fig. 1
Presumed etiologic factors of electrical storm. Cause assignment was made by the physician at the time the electrical storm occurred. CHF, congestive heart failure.

Table 2
Clinical and Laboratory Characteristics of Patients Presenting with Electrical Storm
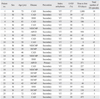
LVEF, left ventricular ejection fraction; ES, electrical storm; CAD, coronary artery disease; SNH, structurally normal heart; NIDCMP, nonischemic dilated cardiomyopathy; ARVD, arrhythmogenic right ventricular dysplasia; HCMP, hypertrophic cardiomyopathy; VT, ventricular tachycardia; VF, ventricular fibrillation.
References
1. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006. 114:e385–e484.
2. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996. 335:1933–1940.

3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002. 346:877–883.

4. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study Cardiac Arrest Study Hamburg. Canadian Implantable Definrillator Study. Eur Heart J. 2000. 21:2071–2078.

5. Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998. 32:1909–1915.

6. Greene M, Newman D, Geist M, Paquette M, Heng D, Dorian P. Is electrical storm in ICD patients the sign of a dying heart? Outcome of patients with clusters of ventricular tachyarrhythmias. Europace. 2000. 2:263–269.

8. Gatzoulis KA, Andrikopoulos GK, Apostolopoulos T, Sotiropoulos E, Zervopoulos G, Antoniou J, et al. Electrical storm is an independent predictor of adverse long-term outcome in the era of implantable defibrillator therapy. Europace. 2005. 7:184–192.

9. Verma A, Kilicaslan F, Marrouche NF, Minor S, Khan M, Wazni O, et al. Prevalence, predictors, and mortality significance of the causative arrhythmia in patients with electrical storm. J Cardiovasc Electrophysiol. 2004. 15:1265–1270.

10. Exner DV, Pinski SL, Wyse DG, Renfroe EG, Follmann D, Gold M, et al. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001. 103:2066–2071.
11. Endoh Y, Ohnishi S, Kasanuki H. Clinical significance of consecutive shocks in patients with left ventricular dysfunction treated with implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 1999. 22:187–191.

12. Pacifico A, Ferlic LL, Cedillo-Salazar FR, Nasir N Jr, Doyle TK, Henry PD. Shocks as predictors of survival in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 1999. 34:204–210.

13. Villacastín J, Almendral J, Arenal A, Albertos J, Ormaetxe J, Peinado R, et al. Incidence and clinical significance of multiple consecutive, appropriate, high-energy discharges in patients with implanted cardioverter-defibrillators. Circulation. 1996. 93:753–762.

14. Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation. 2002. 106:2145–2161.

15. Dorian P. [Etiologies of electric storm]. Arch Mal Coeur Vaiss. 1997. 90:27–31.
16. Kowey PR, Levine JH, Herre JM, Pacifico A, Lindsay BD, Plumb VJ, et al. Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995. 92:3255–3263.

17. Scheinman MM, Levine JH, Cannom DS, Friehling T, Kopelman HA, Chilson DA, et al. Dose-ranging study of intravenous amiodarone in patients with life-threatening ventricular tachyarrhythmias. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995. 92:3264–3272.

18. Israel CW, Barold SS. Electrical storm in patients with an implanted defibrillator: a matter of definition. Ann Noninvasive Electrocardiol. 2007. 12:375–382.

19. Wood MA, Simpson PM, Stambler BS, Herre JM, Bernstein RC, Ellenbogen KA. Long-term temporal patterns of ventricular tachyarrhythmias. Circulation. 1995. 91:2371–2377.

20. Wood MA, Ellenbogen KA, Liebovitch LS. Electrical storm in patients with transvenous implantable cardioverter-defibrillators. J Am Coll Cardiol. 1999. 34:950–951.
21. Sesselberg HW, Moss AJ, McNitt S, Zareba W, Daubert JP, Andrews ML, et al. Ventricular arrhythmia storms in postinfarction patients with implantable defibrillators for primary prevention indications: a MADIT-II substudy. Heart Rhythm. 2007. 4:1395–1402.

22. Stuber T, Eigenmann C, Delacrétaz E. Characteristics and relevance of clustering ventricular arrhythmias in defibrillator recipients. Pacing Clin Electrophysiol. 2005. 28:702–707.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download