Abstract
A liver transplantation is a treatment option in selected patients with hepatocellular carcinoma (HCC). Despite the adequate selection of candidates, recurrences of HCC may still develop. Solitary extrahepatic metastasis from HCC after a liver transplantation is rare. Here we report two cases of HCC demonstrated extrahepatic recurrence to the adrenal gland and spleen, respectively, within one year after a liver transplantation. Since the treatment of solitary extrahepatic metastasis from HCC after a liver transplantation is not standardized, surgical resection was performed. In the case of HCC adrenal metastasis, innumerable intrahepatic metastases were found two months after the adrenalectomy. And 16 months after adrenalectomy, the patient expired due to tumor progression and hepatic failure. In the case of HCC splenic metastasis, postoperative radiation therapy was performed. However, two recurrent HCC nodules were found 15 months after the splenectomy and received transarterial chemoembolization (TACE). And 29 month after the splenectomy, the patient also expired as same causes of former patient.
There are several treatments for hepatocellular carcinoma (HCC), and a liver transplantation can be performed in a selected group of patients as such a treatment. After the introduction of the Milan and University of California at San Francisco (UCSF) criteria, the candidates for a liver transplantation have been expanded. The use of a specific selection criteria has been shown to improve tumor-free survival in patients undergoing a liver transplantation for HCC.1-3 However, despite the adequate selection of candidates, recurrences of HCC may still develop and result in death.4,5 The adrenal gland is a common target organ of hematogenous metastasis from HCC6 however, the spleen has been rarely reported as a metastatic site of HCC. We report two cases of solitary extrahepatic metastasis from HCC after a liver transplantation treated with surgical resection.
A 71-year-old man with HCV hepatitis was diagnosed with HCC in 2002. He was treated with radiofrequency ablation and transarterial chemoembolization (TACE) for the HCC at another hospital. When he was referred to our hospital in December 2005, a CT scan demonstrated innumerable tiny intrahepatic HCC nodules. He belonged to Child-Pugh class A, and the alpha-fetoprotein (AFP) level was measured at 87.96 IU/mL. After a treatment with two cycles of intrahepatic arterial cisplatin and systemic 5-FU chemotherapy, he underwent a cadaver donor liver transplantation in March 2006 in China, although he met neither Milan nor UCSF criteria. He took Pegylated interferon alpha and Tacrolimus after the liver transplantation.
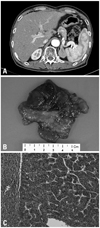
Eleven months post-transplantation, a CT scan revealed a solitary left adrenal mass, 3.5 × 2.3 cm in size, without any other recurrent lesions (Fig. 1A). The laboratory values showed hemoglobin of 10.2 g/dL, WBC of 3,270/µL, platelet count of 119,000/µL, albumin of 4.5 g/dL, AST/ ALT of 32/15 IU/L, total bilirubin of 0.5 mg/dL, prothrombin time of 10.7 sec, and AFP of 6.02 IU/mL. The patient was in a good performance status and underwent a left adrenalectomy with retroperitoneal approach in April 2007. A multinodular grayish-brown, soft-to-friable solid mass was grossly noted. It was well demarcated, measuring 5 × 3.5 cm in size. Tumor emboli were present in the blood vessels connecting to the mass (Fig. 1B). The pathological examination demonstrated metastatic HCC without metastatic lymph nodes retrieved from around the aorta and the left renal vessels (Fig. 1C). There were no immediate complications during recovery. Immunosuppressive therapy (Tacrolimus) continued following the adrenalectomy.
However, a follow-up CT scan taken two months after the adrenalectomy revealed innumerable intrahepatic arterial enhancing nodules consistent with HCC. There were no recurrent lesions at the site of the adrenalectomy. The patient received systemic adriamycin chemotherapy with subsequent transarterial chemoembolization. The intrahepatic recurrent tumors were not controlled, although chemotherapy was performed. The patient expired 16 months after the adrenalectomy due to liver failure.
A 63-year-old man who was a carrier of HBV for 30 years was admitted for a ruptured HCC located in segments VIII of the liver in August 2004. He belonged to Child-Pugh class A. He was treated three consecutive times with TACE. The patient then underwent a central bisectionectomy in February 2005 due to a remaining viable tumor (Fig. 2A). The tumor was 5.7-cm in diameter and the resection margin was tumor-free with a distance of 3.5 cm (Fig. 2B). It was compatible with HCC and was composed of 60% necrosis due to TACE. Vascular invasion was not found. Sixteen months after the operation, another 1.5 cm-sized recurrent HCC on segment VII was found, and the AFP level increased to 54.53 IU/mL. He was treated with TACE however; the remaining viable tumor was still detected with an increase in size. Then the patient underwent a cadaver donor liver transplantation in November 2006 in China. He thereafter took Tacrolimus for immunosuppression. Four months after the liver transplantation, a newly-developed 3 cm-sized heterogeneous mass was found in the hilum of the spleen (Fig. 3A and B). The laboratory values showed hemoglobin of 10.7 g/dL, WBC of 3400/µL, platelet count of 146,000/µL, albumin of 4.4 g/dL, AST/ ALT of 18/9 IU/L, total bilirubin of 0.4 mg/dL, prothrombin time of 11.0 sec, and AFP of 2.15 IU/mL. The patient was closely observed for three months, and a splenectomy was performed in July 2007 due to a mass growth without evidence of intrahepatic recurrence or distant metastasis. A well-defined, white, solid mass measuring 6 × 5 × 4.5 cm was found. It was partially encapsulated and was pathologically compatible with metastatic HCC (Fig. 3C and D). Because the lesion invaded the perisplenic space, the patient received radiation therapy on the splenectomy site following an uneventful postoperative recovery. The radiation dosage was 50.4 Gy. The patient demonstrated no evidence of recurrence for 15 months. The patient received only adjuvant radiation therapy without any other treatment modality. However, a follow-up CT scan taken 15 months after the splenectomy demonstrated two nodular lesions in segment VI of the liver compatible with HCCs. He then received TACE for 4 times. However, he was in the progressive disease status and was expired 29 months after the splenectomy.
A liver transplantation is a treatment option for selected HCC patients with cirrhosis. For those patients with recurrent HCC and poor liver function, a liver transplantation may be a choice to prolong their lives.7 The recurrence of HCC after a liver transplantation has been reported to be about 10%.8 Some authors reported that the recurrences occurring within one year of the liver transplantation are mainly extrahepatic.9 The two cases in this report demonstrated extrahepatic HCC recurrence within one year after a liver transplantation. Although early tumor recurrence is dependent upon many factors, namely vascular invasion and the number and size of the tumor, the influence of the post-transplant immunosuppression in a dysfunctional immune system cannot be excluded as contributing to the acceleration of the recurrence. Although it is known that the pharmacologic immunosuppression required after a transplantation can accelerate tumor growth, the possible influence of different immunosuppressive schedules on HCC recurrence after a liver transplantation has been poorly investigated until recently.10
There is no consensus on the treatment of recurrent HCC following a liver transplantation, and treatment of the recurrent tumor is not always effective. Moreover, early recurrence shows a short survival period; however, in recent years, treatment has been more aggressive in an effort to improve survival.11 Several therapeutic modalities have been proposed such as surgical resection, TACE, percutaneous ethanol injection therapy, and radiation therapy. Aggressive management of extrahepatic metastatic lesions have achieved improved outcome.12,13
The adrenal gland is the second most common site of metastasis from HCC.6 In those cases, an adrenalectomy is considered to be the treatment of choice. However, the survival benefit and disease control effect of an adrenalectomy in the case 1 patient is questionable. It may be better to closely observe the patient and provide systemic chemotherapy or TACE rather than to perform a surgical resection. In a clinical situation, a surgical resection should be carefully considered for a patient with good performance and no intrahepatic recurrence. Surgery for adrenal metastasis from HCC may be indicated not only for patients without evidence of an intrahepatic or extrahepatic lesion, but also for patients with a well-controlled intrahepatic or extrahepatic lesion to increase survival.14
In the cases of solitary splenic metastasis of HCC, it is also difficult to predict the prognosis. Because metastatic splenic tumors are usually detected as a part of disseminated hematogenous metastasis with multi-organ involvement, isolated splenic metastasis is exceedingly rare.14 The survival benefit of a splenectomy for a solitary metastatic splenic tumor is unknown and the disease control effect will be evaluated. However, with more aggressive management of metastatic tumors, the increase to perform splenectomy as a treatment of solitary splenic metastasis during the past decade might be expected to continue.15
In conclusion, the survival benefit of surgical resection in the management of isolated extrahepatic metastases of HCC is questionable due to its rarity. Surgical resection is a treatment option with low morbidity that may offer a chance for long-term survival in selected patients without poor prognostic factors and whose intrahepatic tumors are controlled. Surgical treatment can be an option to treat extrahepatic solitary metastasis from HCC.
Figures and Tables
Fig. 1
A CT scan reveals a newly-developed solitary left adrenal mass, which was 3.5 × 2.3 cm in size, homogenous, and well-demarcated, suggesting HCC metastasis (A). Pathologic specimen following adrenalectomy (B) and microscopic exam, Hematoxylin & Eosin stain, ×100 (C).
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084120).
References
1. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996. 334:693–699.

2. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001. 33:1394–1403.

3. Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004. 127:5 Suppl 1. S268–S276.

4. Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000. 216:698–703.

5. Castroagudín JF, González-Quintela A, Martínez J, Tomé S, Forteza J, Varo E. Bilateral adrenal metastases from hepatocellular carcinoma after liver transplantation. Hepatogastroenterology. 2002. 49:249–251.
6. Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, et al. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer. 1983. 51:863–877.

7. Hu RH, Ho MC, Wu YM, Yu SC, Lee PH. Feasibility of salvage liver transplantation for patients with recurrent hepatocellular carcinoma. Clin Transplant. 2005. 19:175–180.

8. Llovet J, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005. 25:181–200.

9. Pérez-Saborido B, de los Galanes SJ, Menéu-Díaz JC, Romero CJ, Elola-Olaso AM, Suárez YF, et al. Tumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factors. Transplant Proc. 2007. 39:2304–2307.

10. Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005. 11:497–503.

11. Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, et al. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2007. 39:2308–2310.

12. Poon RT, Fan ST, O'uilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002. 195:311–318.

13. Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009. 50:601–612.

14. Park JS, Yoon DS, Kim KS, Choi JS, Lee WJ, Chi HS, et al. What is the best treatment modality for adrenal metastasis from hepatocellular carcinoma? J Surg Oncol. 2007. 96:32–36.

15. Lee SS, Morgenstern L, Phillips EH, Hiatt JR, Margulies DR. Splenectomy for splenic metastases: a changing clinical spectrum. Am Surg. 2000. 66:837–840.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download