Abstract
Bortezomib is an inhibitor of 26S proteasome, which is an effective treatment for multiple myeloma. The common adverse effects of bortezomib are asthenic conditions, gastrointestinal disturbances, and peripheral neuropathy. Here we describe a patient with dyspnea and general weakness because of complete atrioventricular block while receiving bortezomib. We immediately stopped bortezomib, and after inserting a permanent VDD pacemaker, the patients' symptoms disappeared.
Multiple myeloma is a hematologic malignancy characterized by the proliferation of monoclonal plasma cells that produce monoclonal immunoglobulins. Treatments for multiple myeloma have significantly changed with improved understanding of potential myeloma targets, and have led to the development of molecular-targeted antineoplastic agents, such as bortezomib. Bortezomib is an inhibitor of 26S proteasome, which is effective treatment in multiple myeloma. This inhibition makes the accumulation of poly-ubiquitinated proteins involved in a multitude of signaling pathways. Apoptosis is one of the resulting effects, with relative selectivity for malignant cells as opposed to normal cells.1
The common adverse effects of bortezomib are asthenic conditions, gastrointestinal disturbances, and peripheral neuropathy.2 Cardiac rhythm abnormalities secondary to use of bortezomib is uncommon. In this report we describe a multiple myeloma patient who experienced a complete atrioventricular (AV) block while receiving bortezomib with dexamethasone.
A-65-year-old female with no prior cardiovascular history was admitted to the hospital complaining of a sudden onset of weakness and dyspnea. There was no chest pain or palpitation. Her pulse was found to be 53 beats per minute (bpm) and her blood pressure was 110/70 mmHg. The physical examination revealed a split second heart sound with no other abnormalities. The chest X-ray was normal with slight cardiomegaly (Fig. 1). The laboratory tests showed normal kidney and liver function, cardiac enzyme and electrolytes, with normocytic anemia of Hb 9.5 g/dL. The electrocardiogram revealed a complete AV block with premature ventricular block and ventricular rate of 53 bpm (Fig. 2B). Previous electrocardiograms had revealed no conduction abnormalities (Fig. 2A). Holter monitoring also showed complete AV block. The echocardiogram showed both atrial enlargements with normal ventricular systolic function, ejection fraction revealing 65%. A myocardial biopsy was done to distinguish from amyloidosis but revealed no evidence.
The past medical history showed a stage IIA multiple myeloma IgG kappa diagnosed 6 months prior. At diagnosis she had an M-protein of 4.38 g/dL, hemoglobin 9.7 g/dL normocytic anemia and creatinine 0.8 mg/dL, with normal renal function. Her bone marrow aspiration and biopsy revealed 52% of plasma cells. There was neither hypercalcemia nor neuropathy but the patient had a general ache with multiple compression fractures at the spine.
In first line chemotherapy, a regimen of peroral (PO) melphalan 0.15 mg/kg with prednisolone on days 1-7 every four weeks was started. After 3 cycles of melphalan and prednisolone the patient's M-protein decreased by 4.00 g/dL. But she still complained of a general ache and her total proteins were still high. Considering this to be a stable disease state we changed the chemotherapy regimen to IV bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 every 3 weeks and dexamethasone 40 mg PO weekly. The response was good, with decrease of M-protein to 0.66 g/dL and disappearance of general ache after 2nd cycle of bortezomib. When her acute symptomatic bradycardia appeared, she was expected to have a 3rd cycle of bortezomib.
A permanent VDD pacemaker was successfully inserted (Fig. 3), and the symptoms of dyspnea and general weakness completely disappeared. Because bortezomib was thought to be the reason for the AV block, it was immediately stopped and the regimen was changed to thalidomide, cyclophosimide, and dexamethasone regimen.
Cases of cardiac arrhythmias and conduction disorders caused by bortezomib were observed in several clinical trials.3-5 Early studies exploring the safety of prolonged therapy with bortezomib in a relapsed/refractory myeloma setting, Berenson, et al.5 reported a case of complete AV block, and only one case was reported by Dasanu1 to insert permant pacemaker because of a complete AV block secondary to bortezomib use.1 In our patient's case, it is possible that the multiple myeloma itself combined with bortezomib impaired AV conduction in a patient with an underlying AV node problem. But since the patient had neither a pre-existing heart condition nor any type of arrhythmia before receiving bortezomib, we believe that botezomib played a large part occurring complete AV block.
The mechanism of a complete AV block secondary to bortezomib is uncertain. Bortezomib is a dipeptide boronate proteasome inhibitor, which reversibly inhibits the 26S proteasome. But it is postulated that bortezomib affects not only nuclear factor- kB activity but also various signaling pathways its metabolites might cause cellular damage of the heart. Although cardiac toxicity with bortezomib is generally thought to be rare, moderate shortness of breath, palpitations and pedal edema have been documented in patients treated with this agent,6 which demonstrates the possibility that bortezomib could also damage the conduction system of the heart.
In conclusion, cardiac rhythm abnormalities, including complete AV block are rare side effects of bortezomib use, but can be critical to the patient. Thus, early recognition of this side effect and prompt withdrawal of bortezomib is needed to prevent fatal outcomes.
Figures and Tables
 | Fig. 2(A) A 12-lead electrocardiogram of the patient before using bortezomib. No specific ST or T abnormalities, with normal sinus rhythm. (B) A 12-lead electrocardiogram of the patient at admission. More P waves than the QRS complexes and dissociation with P waves and QRS complexes, which stands for complete atrioventricular block. |
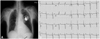 | Fig. 3(A) Chest X-ray after pacemaker insertion. The pacemaker lead is shown at the apex of right ventricle in the X-ray (arrow), which indicates successful pacemaker insertion. (B) A 12-lead electrocardiogram after insertion of pacemaker. The electrocardiogram shows successful pacing of the pacemaker. |
References
1. Dasanu CA. Complete heart block secondary to bortezomib use in multiple myeloma. J Oncol Pharm Pract. 2010. 04. 20. [Epub ahead of print].

2. Colson K, Doss DS, Swift R, Tariman J, Thomas TE. Bortezomib, a newly approved proteasome inhibitor for the treatment of multiple myeloma: nursing implications. Clin J Oncol Nurs. 2004. 8:473–480.

3. Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, et al. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp Neurol. 2007. 204:317–325.

4. Shah MH, Young D, Kindler HL, Webb I, Kleiber B, Wright J, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004. 10:6111–6118.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download