Abstract
Duchenne muscular dystrophy usually affects males. However, females are also affected in rare instances. Approximately 8% of female Duchenne muscular dystrophy (DMD) carriers are manifesting carriers and have muscle weakness to some extent. We investigated the clinical features of 3 female patients with dystrophinopathy diagnosed by clinical, pathological, and genetic studies at our neuromuscular disease clinic. The onset age of manifesting symptoms varied (8-28 years). Muscle weakness grade varied as follows: patient 1 showed asymmetrical bilateral proximal upper and lower extremities weakness, patient 2 showed asymmetrical bilateral upper extremities weakness similar to scapulohumoral muscular dystrophy, and patient 3 had only bilateral asymmetric proximal lower extremities weakness. Two patients had familial histories of DMD (their sons were diagnosed with DMD), but the 1 remaining patient had no familial history of DMD. The serum creatine kinase level was elevated in all patients, but it was not correlated with muscular weakness. An electromyography study showed findings of myopathy in all patients. One patient was diagnosed with a DMD carrier by a muscle biopsy with an immunohistochemical stain (dystrophin). The remaining 2 patients with familial history of DMD were diagnosed by multiplex ligation-dependent probe amplification (MLPA). There were inconsistent clinical features in the female carriers. An immunohistochemical analysis of dystrophin could be useful for female carrier patients. Also, multiplex ligation-dependent probe amplification is essential for the diagnosis of a manifesting female carrier DMD in female myopathic patients because conventional multiplex PCR could not detect the duplication and is less accurate compared to MLPA.
Two-thirds of mothers of affected males are thought to be Duchenne muscular dystrophy (DMD) gene carriers and approximately 8% of female DMD carriers have muscle weakness to some extent and are designated as manifesting DMD carriers.1-3 We investigated the clinical features of 3 female myopathic carrier patients with DMD.
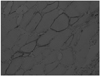
A 9 year-old girl was admitted with complaints of slow, progressive proximal limb weakness that started 1 year prior. Until the age of 8, she had grown up without abnormality, developed a normal walking course, and normal cognitive function. There was no familial history of neuromuscular disease, including her elder brother. Upon neurological examination, she showed normal intelligence and no cranial nerve abnormality. However, her bilateral limb-girdle muscular power decreased (right arm abductor, arm elevator, arm adductor, forearm flexor, forearm extensor G4+/left arm abductor, arm elevator, arm adductor, forearm flexor, forearm extensor G4°/right hip flexor, hip extensor, knee flexor, knee extensor G4°/left hip flexor, hip extensor, knee flexor, knee extensor G4+). Gower signs and bilateral calf muscle pseudohypertrophy were also observed. Liver enzymes, serum CK, lactate dehydrogenase, and aldolase were elevated (AST: 97 IU/L; ALT: 225 IU/L; and CK: 456 1 U/L, CK-MB 225 U/L, LDH 1714 IU/L, and Aldolase 16.4 U/mL). The patient also showed normal findings for viral hepatitis markers and arterial blood gas analysis. Electrocardiography and cardiac echocardiography showed no abnormal findings. An electromyography study revealed spontaneous activity (positive sharp waves) and short duration polyphasic potentials in voluntary contraction. We carried out a muscle biopsy on the left vastus lateralis muscle because limb-girdle muscular dystrophy was suspected as the patient was female and did not have a familial history of DMD. The expression of dystrophin protein showed mosaic patterns on a dystrophin immunohistochemical stain from a muscle biopsy (Fig. 1). The other immunohistochemical stain for detecting limb girdle muscular dystrophy and congenital myopathy revealed no abnormality. Accordingly, she was diagnosed as a DMD carrier.
A 32 year-old woman was admitted with complaints of progressive limb weakness that started 9 years prior. She had 2 sons who were diagnosed by exon duplication (exon 52, 53, 56-61) with DMD. On a neurological examination, she showed intellectual disability (IQ = 70) and her bilateral upper extremities, especially scapulohumoral lesion, showed muscular weakness (right arm abductor, elevator, adductor G4°/left arm abductor, elevator, adductor G4+). The power of other muscles was within a relatively normal range. Gower signs and bilateral calf pseudohypertrophy were not observed. Serum CK was mildly elevated (CK: 3708 U/L). Cardiac echocardiography showed normal findings, but ECG findings revealed the R/S > 1 in Vl, 2 leads, and deep Q wave in I, V4-6 leads. An electromyography study revealed spontaneous activity (fibrillation and positive sharp waves) and short duration polyphasic potentials in voluntary contraction. A conventional multiplex polymerase chain reaction (PCR) had negative findings, but a multiplex ligation-dependent probe amplification (MLPA) revealed exon duplication at exon 52, 53, 56-61.
A 34 year-old woman was admitted with complaints of progressive weakness in both legs that started 6 years prior. She had 2 sons, who were diagnosed by exon deletion (exon 43, 44 and 45) with DMD. On a neurological examination, she showed intellectual disability (IQ = 78) and her bilateral lower extremities showed muscular weakness (right hip flexor, hip extensor, knee flexor, knee extensor G3°/left hip flexor, hip extensor, knee flexor, knee extensor G4-). The power of the other muscles was within a relatively normal range. Gower signs and bilateral calf pseudohypertrophy were not observed. Serum CK was mildly elevated (CK: 1289 U/L). Electrocardiography and cardiac echocardiography showed no abnormal findings. An electromyography study revealed spontaneous activity (fibrillation and positive sharp waves) and short duration polyphasic potentials in voluntary contraction. A conventional multiplex PCR had negative findings but a MLPA revealed exon deletion at 43, 44 and 45.
The clinical features of female carriers in this study were inconsistent. In Duchenne and Becker dystrophinopathic carriers, muscular weakness is predominantly asymmetric (81.8%). 41% of dystrophinopathic carriers have weakness limited to the upper extremities, 23% of carriers have weakness of the lower extremities, and 36% of carriers have muscular weakness of the upper and lower extremities.4 In this study, the patients revealed asymmetrical muscular weakness in different lesion.
Serum CK levels are elevated in approximately 45-70% of carriers and the measurement of serum CK is the most commonly used method for detecting carriers.5 In our cases, serum CK levels were elevated in all patients. But, although muscular weakness was more severe in the third case, the serum CK level was lowest. The CK level is usually due to the progressive involvement of muscle function. Further study regarding the correlation between the serum CK level and muscular weakness is requested in female carriers. In DMD carriers, incidences of cardiac involvement progresses with age. Researchers reported latent and clinical cardiac involvement in 55% of carriers under the age of 16 and in 90% of carriers after the age of 16, especially dilated cardiomyopathy.6 Abnormal ECG patterns were reported (R/S > 1 in Vl, 2: deep Q wave in I, V4-6) in 6.6-16.44% of female carriers of DMD.7 In this case, ECG findings of the second case were compatible with the previous report. However, ECG and echocardiographs of the other patients showed whole normal findings.
The dystrophin is localized on the cytoplasmic surface of the plasma membrane of skeletal and cardiac muscle cells.8 The mosaic distribution of dystrophin-positive and -negative fibers in the skeletal and cardiac muscles of biopsy is the characteristic finding of a DMD carrier.9 The surface membranes of muscles in DMD patients do not react with anti-dystrophin antiserum, while those from carriers show a mosaic pattern.9 In the first case, we could diagnosis the DMD manifesting carrier because the patient's biopsy revealed a typical mosaic pattern in immunohistochemical stains. Therefore, the dystrophin immunohistochemistry could be useful to diagnosis what type of myopathy is present and whether mosaicism is present in female myopathic patients.
The multiplex PCR have traditionally been used in a clinical diagnostic set up and allow a detection of 90-95% of the deletions in male patients, but duplications are not identified in DMD.10-12 However, the MLPA method has turned out to be reliable and accurate for identifying duplications and deletions in DMD.13,14 The MLPA technique could increase mutation pick-up rate by 33% rather than PCR.14 Moreover, the technique could confidently identify carrier individuals.14 In our cases, exon duplication detected in case 2, on the other hand, exon deletion also detected in case 3 but the PCR could not detect genetic abnormality. These results support that MLPA is essential for diagnosis of manifesting female carrier DMD in female myopathic patients because conventional multiplex PCR could not detect the duplication and is less accurate compared to MLPA.
Figures and Tables
References
1. Walcher T, Kunze M, Steinbach P, Sperfeld AD, Burgstahler C, Hombach V, et al. Cardiac involvement in a female carrier of Duchenne muscular dystrophy. Int J Cardiol. 2010. 138:302–305.

2. Ceulemans BP, Storm K, Reyniers E Jr, Callewaert L, Martin JJ. Muscle pain as the only presenting symptom in a girl with dystrophinopathy. Pediatr Neurol. 2008. 38:64–66.
3. Hoffman EP, Arahata K, Minetti C, Bonilla E, Rowland LP. Dystrophinopathy in isolated cases of myopathy in females. Neurology. 1992. 42:967–975.
4. Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, et al. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999. 353:2116–2119.

5. Griggs RC, Mendell JR, Brooke MH, Fenichel GM, Miller JP, Province M, et al. Clinical investigation in Duchenne dystrophy: V. Use of creatine kinase and pyruvate kinase in carrier detection. Muscle Nerve. 1985. 8:60–67.

6. Politano L, Nigro V, Nigro G, Petretta VR, Passamano L, Papparella S, et al. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA. 1996. 275:1335–1338.

7. Lukasik E. Electrocardiographic studies in female carriers of Duchenne muscular dystrophy. J Neurol. 1975. 209:279–285.

8. Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987. 51:919–928.

9. Arahata K, Ishihara T, Kamakura K, Tsukahara T, Ishiura S, Baba C, et al. Mosaic expression of dystrophin in symptomatic carriers of Duchenne's muscular dystrophy. N Engl J Med. 1989. 320:138–142.

10. Chamberlain JS, Gibbs RA, Ranier JE, Nguyen PN, Caskey CT. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988. 16:11141–11156.

11. Beggs AH, Koenig M, Boyce FM, Kunkel LM. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990. 86:45–48.
12. Kunkel LM, Snyder JR, Beggs AH, Boyce FM, Feener CA. Lindsten J, Petterson U, editors. Searching for dystrophin gene deletions in patients with atypical presentations. Etiology of Human Diseases at the DNA Level. 1991. New York: Raven Press;51–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download