Abstract
Purpose
Radiotherapy for head and neck cancer does not impair the voice quality as much as laser treatment or surgery, but it can induce muscle wasting and fibrosis and symptoms of dry mouth. We investigated the effect of irradiation on the myosin heavy chain (MyHC) expression in laryngeal muscles.
Materials and Methods
Rats were irradiated with one dose of 10, 15, 20, 25, 30, or 35 Gy and other rats were irradiated with 20 Gy. The thyroarytenoid (TA), posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles were subjected to reverse transcription-polymerase chain reaction (RT-PCR).
Results
Two weeks after irradiation with 10, 15, or 20 Gy, all the MyHC type expressions had decreased in a dose-dependent manner in the TA, PCA, and CT muscles, and especially the expression of MyHC IIa decreased much more than the expressions of the other MyHC isoforms in all muscles. In the 20 Gy-irradiated rats, almost all the MyHC isoform expressions declined over 12 weeks in the TA, PCA, and CT muscles, except for the MyHC I expression in the PCA and CT muscle. The MyHC IIa expression was markedly decreased in all the muscles.
Conclusion
The laryngeal muscles responded differently to radiation, but they showed a time-dependent and long-lasting decrease in the expressions of all the MyHC isoforms in the TA, PCA, and CT muscles. In particular, the expression of the MyHC IIa isoform in all the muscles may be more sensitive to irradiation than the expressions of the other MyHC isoforms.
Early stage laryngeal cancer can be treated with radiation therapy or excision of the lesion with using a laser,1,2 but laser treatment generally negatively affects the voice quality because it removes part of the vocal cord. In contrast, radiation therapy, which completely cures 90% of the cases of early stage laryngeal cancer, generally well preserves the voice of the patient. However, some irradiated patients do experience voice changes because the radiation destroys the salivary gland, resulting in xerostomia, and the radiation causes muscle wasting and fibrosis and it may change the composition of the myosin heavy chain (MyHC) isoforms.3,4 There is currently a great deal of active research on MyHC isoforms in the field of otorhinolaryngology.4-6 These studies have provided a good foundation for analyzing the changes of the laryngeal muscles' expression of MyHC isoforms after irradiation.
MyHC is an important factor that controls the pattern of muscle contraction. There are a number of MyHC isoforms. Of these, MyHC type I is expressed in muscles with a slow contraction speed while MyHC type II is primarily involved in fast muscle contraction. In particular, MyHC type IIb is involved in very fast muscle contraction, although it is associated with a severe fatigue phenomenon. In contrast, IIa is involved in slower muscle contraction and it is associated with a less severe fatigue phenomenon, while IIx participates in intermediate muscle contraction.7,8 Furthermore, a recent study has shown that MyHC type IIeo, which is present in the extraocular muscles of the eye, is involved in the fastest muscle contraction.6
We examined the change in body weight and the change of the MyHC isoform expression in a murine model according to different radiation doses and over time after irradiation. To accomplish this, the thyroarytenoid (TA), the posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles of rats were dissected out and examined by SYBR green real time-reverse transcription-polymerase chain reaction (RT-PCR).
Fifty-five Sprague-Dawley (SD) rats (Samtako Co., O San, Korea) (mean age: 7 to 8 weeks, mean weight: 220 ± 30 g) were maintained at three rats per cage and they were given free access to standard rat chow and water. The rats were kept at a room temperature of 23 ± 1℃ and humidity of 55 ± 5%, with 12 hour lighting at 150-300 lux.
Our experiment is composed of two subexperiments. In the first experiment, 35 rats were used, and these were divided into seven groups according to a single radiation dose: the control (no radiation), 10 Gy, 15 Gy, 20 Gy, 25 Gy, 30 Gy, and 35 Gy groups of 5 rats per group. The rats were sacrificed 2 weeks later after X-irradiation by a barbiturate overdose. In the second experiment, 20 rats were exposed to 20 Gy, and they were divided into four groups of 5 rats each according to the elapsed time after radiation exposure: 3 days, 2 weeks, 4 weeks, and 12 weeks. Five rats of each group were also sacrificed by a barbiturate overdose to examine the effects of radiation on the body weight loss and the laryngeal muscles' MyHC isoform expression.
The head and neck areas of the rats were evenly irradiated with 2 Gy per minute by using a 6 MV energy therapeutic linear accelerator (CLINAC 600C, Varian, Palo Alto, CA, USA). To irradiate the head and neck area with a sufficient dose of radiation, a 1.0 cm bolus was used for increasing the radiation dose. A single dose of irradiation was administered. To immobilize the animals during their irradiation, the rats were anesthetized with an intraperitoneal injection of ketamin hydrochloride (40 mg/kg) and xylazine hydrochloride (5 mg/kg). No side effects or complications occurred during the rats' anesthesia.
Thirty-five rats were grouped into seven groups: the control group and the 10, 15, 20, 25, 30, and 35 Gy groups. Each group was composed of 5 rats. To immobilize the animals during their irradiation, the rats were anesthetized with an intraperitoneal injection of ketamin hydrochloride (40 mg/kg) and xylazine hydrochloride (5 mg/kg). All the animals were sacrificed two weeks later with a barbiturate overdose.
Twenty rats were equally grouped into four groups, and each group was irradiated with 20 Gy of X-ray. The rats in each group were anesthetized and sacrificed 3 days, 2 weeks, 4 weeks, and 12 weeks later, respectively. In general, we examined MyHC types I, IIa, IIb, IIx, and IIL in the TA, PCA, and CT muscles. We did not examine the effect of radiation on the TA expression of MyHC I because it has been reported that the TA muscle expresses very low levels of MyHC I.9 We also did not examine the expression of MyHC IIL in the CT muscle since its baseline expression of this isoform is very low.10
After resection of the larynx from the sacrificed rats by using a surgical microscope, the TA, PCA, and CT muscles were removed and stored in a - 80℃ freezer. The RNA was extracted on the same day.
The total RNA was extracted by using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the method suggested by the manufacturer. The cDNA was synthesized from the RNA, by using Superscript III reverse transcriptase (Invitrogen), and RT-PCR was performed in a total reaction volume of 20 µL. In the first step, a mixture consisting of 1 µL random hexomer, 1 µL oligo (dT) 18, 1 µL 10 mM dNTP mix, 8 µL extracted total RNA and 2 µL DNase-RNase free water was reacted for 5 minutes at 65℃ and then it was cooled on ice for at least 1 minute. Thereafter, 4 µL of 5X first strand buffer, 1 µL of 0.1 M DDT, 1 µL Superscript III RT, and 1 µL RNase OUT (Invitrogen, Madison, WI, USA) were added. The mixture reacted at 25℃ for 10 minutes, 42℃ for 50 minutes, and 85℃ for 5 minutes. The residual complementary RNA was removed by adding 1 µL containing 2 units of E. coli RNAse H (New England BioLabs, Ipswich, MA, USA). After a reaction at 37℃ for 20 minutes, the samples were stored at -20℃ until use. The oligonucleotide primers for the MyHC type I, IIa, IIb, IIx, and IIL isoforms were synthesized (Table 1) by applying the Primer Express Program (Iowa city, IA, USA)which was published by the NCBI, to the MyHC gene sequences of the SD rats. Primers for the housekeeping control gene, i.e., glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were also used. All the primers we used were purchased from Bioneer Co. (Daejeon, Korea). Quantitative PCR was performed by using LightCycler® Software Version 4.0 and the LightCycler® Instrument (Roche, Seoul, Korea). A 20 µL total volume was prepared with 2 µL LightCycler® FastStart DNA Master SYBR Green I, 2.4 µL 25 mM MgCl2, 1 µL of each forward and reverse primer, and 2 µL cDNA and 11.6 µL DNase-RNase free water. For amplification, the reaction mixtures were placed on 32-well optical reaction plates and 50 cycles were performed. After the last cycle, melting curve analysis was performed to examine whether the products were the correct products. The LightCycler® (LightCycler® FastStart DNA Master SYBR Green I) PCR parameters are as follows: preincubation at 95℃ for 10 min, amplification, denaturation at 95℃ for 10 sec, annealing at 58℃ for 5 sec, and extension at 72℃ for 11 sec. The PCR parameters for the melting curve analysis are as follows: denaturation at 95℃ for 0 sec, annealing at 65℃ for 15 sec, melting at 95℃ for 0 sec, and cooling at 40℃ for 30 sec.
The comparative threshold method was applied to examine the effect of radiation on the MyHC expression in the laryngeal muscles of the SD rats. This method assesses the relative expression of the gene of interest by determining the difference between the threshold cycles of the gene of interest and the housekeeping gene. GAPDH served as the housekeeping gene. The 2-ddCt method is well-known as a comparative threshold method and thus the following equation was used: ddCt = control group (Ct target-Ct reference) - irradiated group (Ct target-Ct reference). Two statistical analyses were performed for assessing the MyHC isoform expression. Paired t-tests with Bonferroni's correction was used to analyze the effect of different radiation doses and over time in the each MyHC isoform, and repeated measures of ANOVA (post-hoc analysis by Turkey) were used to analyze the differences between the MyHC isoforms in each muscle. p values less than 0.05 were considered statistically significant.
The rats exposed to 10-20 Gy lost weight soon after irradiation and they gradually recovered the weight lost. The rats exposed to 25 Gy or more radiation continuously lost weight before dying. In particular, the effect of 20 Gy radiation on the living rats was the most severe. These rats could not see properly and they had trouble opening their mouth to eat, so all the rats exhibited weight loss during the first 10 days of the experiment. However, they began to recover their weight after 2 weeks. The rats irradiated more than 20 Gy died between days 7 and 12 and they could not participate in the remainder of the study.
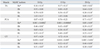
The TA, PCA, and CT muscles of the rats were dissected out separately and subjected to real time RT-PCR for assessing the expressions of the MyHC I, IIa, IIb, IIx, and IIL isoforms. Of the rats irradiated with 10, 15, or 20 Gy, most of the MyHC isoform expressions clearly showed a dose-dependent decrease, excluding the MyHC IIa expression in the TA and PCA muscles (Fig. 1). The statistical analysis using repeated measures of ANOVA (post-hoc analysis by Turkey) revealed that the MyHC IIa expression in particular significantly decreased, as compared to the expressions of the other MyHC isoforms, in all three muscles by 10 to 20 Gy radiation (p < 0.05) (Table 2).
The surviving 20 Gy-irradiated rats were examined 3 days or 2, 4, or 12 weeks after irradiation. The expression of almost all the MyHC isoforms by the TA, PCA, and CT muscles gradually decreased over time, except the MyHC I expression in the PCA and CT muscles and the MyHC IIL expression in the PCA muscle (Fig. 2). The TA, PCA, and CT muscle profiles were similar to the MyHC IIa expression as it rapidly declined from day 3 onwards and continued to decrease for up to 12 weeks. The statistical analysis using repeated measures of ANOVA (post-hoc analysis by Turkey) revealed that the MyHC IIa expression significantly decreased, as compared to the expressions of the other MyHC isoforms (p < 0.05) (Table 3).
Recent studies have investigated the MyHC isoforms that are expressed by rodent and human laryngeal muscles, while others studies have examined the effect of thyroid hormone modulation or denervation and reinnervation on the laryngeal MyHC isoform expression profiles.10-13 Here, we examined the effect of radiation therapy on these expression profiles.
We first assessed the effect of different radiation doses. The radiation was delivered as a single dose so as to limit anesthesia-induced death. The rats were sacrificed 14 days later because it has been reported that acute side effects develop within 2 weeks of irradiation, while chronic side effects start at about 2 weeks.3 In this study, we obtained results similar to Nagler's study. In the second experiment, 20 Gy was delivered as a single dose and the laryngeal muscles were examined at varying intervals after irradiation. The 20 Gy dose was chosen for this experiment because irradiation of 20 Gy to rats is the biological dose equivalent of irradiation of 60 Gy to humans.14,15
For the effect of the radiation dose, almost all the MyHC isoform expressions decreased, according to a radiation dose from 10 up to 20 Gy, in all the TA, PCA, and CT muscles, and especially, the MyHC IIa expression was markedly decreased in all three muscles. The statistical analysis using repeated measures of ANOVA (post-hoc analysis by Turkey) revealed that the MyHC IIa expression was significantly decreased compared to the expressions of the other MyHC isoforms (p < 0.05) (Tables 2 and 3). The expressions of almost all the MyHC isoforms in the TA, PCA, and CT muscles also gradually decreased over time. The TA, PCA, and CT muscle profiles were similar as the MyHC IIa expression declined rapidly from day 3 onwards and it continued to decrease for up to 12 weeks. But the decreasing patterns of all the MyHC isoforms' expressions showed little difference. So, it appears that while radiation generally decreases the laryngeal muscle expression of almost all the MyHC isoforms, the various muscles differ in their response to radiation, and perhaps because they vary in muscle function. There have not been any previous studies that have focused on the change of the MyHC isoform expression in the laryngeal muscle after X-ray irradiation. According to a study on the change of the MyHC isoform expression in rat leg muscle after irradiation, the expressions of MyHC I and IIa in the plantaris muscle increased after 25 Gy irradiation, but the expressions of MyHC IIb and IIx decreased. It was also reported that in the soleus muscle, the expression of embryogenic MyHC decreased after 3,000 rad exposure.5,16
In summary, the laryngeal muscles responded differently to radiation, but they showed a time-dependent and long-lasting decrease in the expression of almost all the MyHC isoforms. We also observed that the MyHC IIa expression decreased more noticeably in the TA, PCA, and CT muscles than the expressions of the other MyHC isoforms, which suggests that the MyHC IIa isoform may be more sensitive to irradiation than the other MyHC isoforms.
Figures and Tables
Fig. 1
MyHC isoform expression changes 2 weeks after irradiation with 10, 15, or 20 Gy. (A) Thyroarytenoid muscle. (B) Posterior cricoarytenoid muscle. (C) Cricothyroid muscle (Bar represent mean value and standard error of the mean, *paired t-test by bonferroni correction). MyHC, myosin heavy chain.

Fig. 2
MyHC isoform expression changes 3 days or 2, 4, or 12 weeks after irradiation with 20 Gy. (A) Thyroarytenoid muscle. (B) Posterior cricoarytenoid muscle. (C) Cricothyroid muscle (Bar represent mean value and standard error of the mean, *paired t-test by bonferroni correction). MyHC, myosin heavy chain.

Table 2
Statistical Analysis of Effect of Different Radiation Doses on the Laryngeal Muscles' MyHC Isoform Expressions (Mean ± SD)
ACKNOWLEDGEMENTS
This work was supported by a Korea Research Foundation Grant funded by the Korea Government (MOEHRD) (KRF-2006-331-E00235) and the Clinical Research Fund of Gyeongsang National University Hospital for the year 2006.
References
1. Cohen SM, Garrett CG, Dupont WD, Ossoff RH, Courey MS. Voice-related quality of life in T1 glottic cancer: irradiation versus endoscopic excision. Ann Otol Rhinol Laryngol. 2006. 115:581–586.

2. Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004. 100:1786–1792.
3. Nagler RM. Extended-term effects of head and neck irradiation in a rodent. Eur J Cancer. 2001. 37:1938–1945.
4. Gillette EL, Mahler PA, Powers BE, Gillette SM, Vujaskovic Z. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995. 31:1309–1318.
5. Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002. 283:C1182–C1195.

6. Shiotani A, Flint PW. Expression of extraocular-superfast-myosin heavy chain in rat laryngeal muscles. Neuroreport. 1998. 9:3639–3642.

7. Shiotani A, Westra WH, Flint PW. Myosin heavy chain composition in human laryngeal muscles. Laryngoscope. 1999. 109:1521–1524.

8. Toniolo L, Maccatrozzo L, Patruno M, Pavan E, Caliaro F, Rossi R, et al. Fiber types in canine muscles: myosin isoform expression and functional characterization. Am J Physiol Cell Physiol. 2007. 292:C1915–C1926.

9. Sokoloff AJ, Li H, Burkholder TJ. Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve. 2007. 36:183–189.

11. Wu YZ, Baker MJ, Crumley RL, Caiozzo VJ. Single-fiber myosin heavy-chain isoform composition of rodent laryngeal muscle: modulation by thyroid hormone. Arch Otolaryngol Head Neck Surg. 2000. 126:874–880.

12. Li ZB, Lehar M, Nakagawa H, Hoh JF, Flint PW. Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg. 2004. 130:217–222.

13. Rhee HS, Lucas CA, Hoh JF. Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem. 2004. 52:581–590.

14. Dubin MG, Feldman M, Ibrahim HZ, Tufano R, Evans SM, Rosenthal D, et al. Allograft dermal implant (AlloDerm) in a previously irradiated field. Laryngoscope. 2000. 110:934–937.

15. Potten CS, Booth D, Cragg NJ, Tudor GL, O'Shea JA, Booth C, et al. Cell kinetic studies in the murine ventral tongue epithelium: mucositis induced by radiation and its protection by pretreatment with keratinocyte growth factor (KGF). Cell Prolif. 2002. 35:Suppl 1. 32–47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download