Abstract
Purpose
To investigate the effect of bevacizumab (Avastin; Genentech, San Francisco, CA, USA) on vascular endothelial growth factor (VEGF) expression and inflammation in fibrovascular membranes in patients with proliferative diabetic retinopathy (PDR).
Materials and Methods
Fibrovascular membranes from 19 eyes of 18 patients with PDR were studied using immunohistochemistry and analyzed in the following 3 groups; group 1: 4 inactive PDR eyes, group 2: 10 active PDR eyes treated preoperatively with adjunctive intravitreal bevacizumab, group 3: five active PDR eyes not treated preoperatively with bevacizumab. Immunohistochemical staining for VEGF, CD31 and CD68 were done.
Results
The immunoreactivity to VEGF and CD 31-positive blood vessels was significantly higher in membranes from group 3 than group 1 (p = 0.007 for VEGF, 0.013 for CD 31-positive vessels). Intravitreal bevacizumab caused a reduction in VEGF expression and vascular densities in 4 out of 10 (40%) excised membranes from eyes with PDR. However, six membranes (60%) in group 2 still demonstrated relatively strong VEGF expression and high vascular density. Infiltration of macrophages was observed in 16 out of the 19 membranes, and the density of macrophages was increased in group 2 compared with group 1 (p = 0.043).
Conclusion
Intravitreal bevacizumab injections caused some reduction in VEGF expression and vascular densities in a limited number of active PDR patients. A single intravitreal bevacizumab injection may not be enough to induce complete blockage of VEGF and pathologic neovascularization in active PDR patients. Repeated injections, panretinal photocoagulation and/or PPV may be necessary following intravitreal bevacizumab to reinforce the anti-VEGF effect of the drug.
Proliferative diabetic retinopathy (PDR) is characterized by a pathological growth of new blood vessels that often results in catastrophic loss of vision due to vitreous hemorrhage and/or tractional retinal detachment (TRD).
Vascular endothelial growth factor (VEGF) is an angiogenic mitogen, and the involvement of VEGF in PDR has been suggested by studies that have demonstrated that the vitreous humor from eyes with PDR contains a large amount of VEGF,1,2 and that VEGF is expressed in fibrovascular tissues obtained from PDR patients.3-5 The discovery and cloning of VEGF in 1989 and subsequent development of antibodies to it allowed for the identification of VEGF's key role in the development of retinal neovascularization in PDR.6,7 In addition to the key role of VEGF in the development of PDR, there is evidence that chronic inflammation may also be involved, as several studies have shown elevated levels of inflammatory markers in patients with diabetic retinopathy.8-10
Bevacizumab (Avastin, Genentech, San Francisco, CA, USA) is a humanized recombinant antibody that binds to most isoforms of VEGF.7 Recent reports have shown that intravitreal bevacizumab is well-tolerated in the short term for treatment of PDR.11-13 Beneficial effects of intravitreal bevacizumab as a preoperative adjunct for TRD in PDR has been reported.14,15 Previous studies have also demonstrated that intravitreal injection of bevacizumab in PDR patients significantly reduces the level of VEGF in aqueous humor.16-18 Although a reduction of the VEGF level in aqueous humor and the regression of fibrovascular proliferation have been observed after clinical treatment intravitreal bevacizumab, the effect of bevacizumab on retinal neovascular membranes is unknown.
In this study, we investigated the clinicohistopathology of surgically excised fibrovascular membranes from PDR patients treated with an intravitreal bevacizumab injection prior to surgery. The expression of VEGF was determined by immunostaining, and the level of vascularization was evaluated by immunodetection of the endothelial marker CD31. The inflammatory infiltration was evaluated using an antibody against CD68 as a marker for macrophages.
Fibrovascular membranes were obtained from 19 eyes of 18 patients during vitrectomy at Yonsei University Eye & Ear, Nose, and Throat Hospital vitreoretinal service, between 1 August 2006 and 28 February 2007. The study followed the tenets of Declaration of Helsinki and was approved by the local Institutional Review Board. Signed and written informed consent was obtained from each patient.
The surgically removed fibrovascular membranes were classified into those with inactive (group 1) and active neovascularization. Neovascularization was considered to be active if the new vessels were perfused and had multibranched preretinal capillaries, and considered to be inactive if a previously documented active proliferation had regressed, or if gliotic vessels or fibrosis were prominent. Of 15 active PDR eyes, 10 eyes had received an intravitreal injection of 1.25 mg bevacizumab one week before planned pars plana vitrectomy (PPV) (group 2), and 5 eyes had received PPV only (group 3).
Ophthalmic examinations, including visual acuity and intraocular pressure measurement, slit lamp examination, dilated fundus examination, fluorescein angiography and color fundus photography, were performed at baseline. The patients' clinical data are summarized in Table 1.
The surgically excised proliferative fibrovascular membranes were fixated in 10% formalin solution in the operating room and embedded in paraffin. Sections, 4 µm thick, were deparaffinized and prepared for immunohistochemical study and hematoxylin-eosin (H&E) staining.
Immunohistochemical analysis was performed using the indirect immunoperoxidase staining technique. Endogenous peroxidase was blocked by methanol/hydrogen peroxide. The poly-clonal rabbit anti-VEGF antibody (Santa Cruz, CA, USA) was diluted (1 : 300) in a 0.05-mol/L concentration of tris (hydroxymethyl) aminomethane-buffered saline (pH 7.5) plus 1% bovine serum albumin and applied for 60 minutes. After washing, the slides were incubated with biotinylated goat antirabbit IgG for 30 minutes, washed and exposed to avidin-peroxidase complex. Three-amino-9-ethylcarbazole (AEC) was used as the chromogen. The slides were counter-stained with Mayer hematoxylin. Observations of the immunostaining were made under a light microscope. When the specimens were too small to provide sufficient sections for all the immunostaining procedures, they were discarded from the study. Omission of the primary antibody in a matched serial epiretinal membrane section, which lacks the specific staining of the respective primary antibody, was used as a negative control.
To study endothelial cell and macrophage distribution, monoclonal mouse antibodies against CD31 (1 : 100 dilution; DakoCytomation, Glostrup, Denmark) and CD68 (1 : 100 dilution; DakoCytomation, Glostrup, Denmark) were used to visualize endothelial cells and macrophages, respectively. Counting of CD31 and 68-positive cells and immunoreactivity scoring for VEGF were performed by an independent observer (JSK) with no knowledge of the patients' clinical information.
The immunoreactivity for VEGF was analyzed and semiquantitatively scored on a scale from 0 to 3 depending on the staining intensity as follows: 0) no detectable staining in the specimen; 1) weak; 2) intermediate; 3) strong.
Immunohistochemical staining of vascular densities for CD31, which stains vascular endothelial cells, was conducted.19 The number of CD31-positive vessels were counted at ×400 power magnification covering the whole stromal area of the membrane and expressed as the average of the three highest readings. Any endothelial cells or clusters that were stained brown and clearly separated from the connective tissue elements were considered as single countable microvessels. The number of CD68-positive cells was counted at ×400 power magnification covering the whole stromal area of the membrane and expressed as the average of the three highest readings.
The baseline demographic and clinical parameters were compared between treatment groups using a Kruskal-Wallis variance analysis for continuous variables and chi-squared tests for categorical variables. Comparisons of study endpoints using comparisons between groups employed the Nemenyi-Damico-Wolfe-Dunn test. Statistical analyses were performed with the R (2.8.1) statistics program. The level of statistical significance was set at p < 0.05, and the results are expressed as mean ± standard deviation.
The specimens from group 1 had sparse vascularized tissue with abundant extracellular matrix and fibrosis. The active neovascular membranes from group 3 were composed of highly vascularized fibrovascular tissue, and the vessels were surrounded by a loosely coherent extracellular matrix. Four out of 10 specimens from group 2 demonstrated regressed vascular channels, with the remaining six specimens showing active PDR characteristics including highly vascularized tissue. The histopathological findings correlated well with the clinical findings in the four eyes with regressed active PDR, with the membranes composed of large caliber vessels and fibroglial tissue (Fig. 1).
The results of immunohistochemical staining are summarized in Table 2. Immunoreactivity to VEGF was detected in the endothelial cells of newly formed vessels in the excised fibrovascular membranes. The immunoreactivity to VEGF was 0.5 ± 0.6 in group 1, 2.0 ± 0.9 in group 2 and 2.6 ± 0.6 in group 3 (Fig. 2). The numbers of CD31-positive blood vessels were 1.3 ± 2.5 in group 1, 11.6 ± 8.4 in group 2 and 17.0 ± 10.4 in group 3 (Fig. 3). The immunoreactivity to VEGF and the number of CD31-positive blood vessels were significantly higher in membranes from group 3 than those from group 1 (p = 0.007 for VEGF, 0.013 for CD 31-positive vessels, Nemenyi-Damico-Wolfe-Dunn test). Intravitreal bevacizumab caused a reduction in VEGF expression and vascular densities in four out of 10 eyes (40%) in group 2, and the histopathological findings in the excised membranes showed regressed PDR (Figs. 1B, 2B, and 3B). However, six eyes (60%) in group 2 exhibited strong VEGF expression and high vascular densities even after the intravitreal bevacizumab injections (Figs. 1C, 2C, and 3C). Infiltration of CD68-positive macrophages was observed in 16 out of 19 membranes. The number of CD68-positive macrophages was 1.5 ± 2.4 in group 1, 18.5 ± 8.4 in group 2 and 8.8 ± 5.7 in group 3 (Fig. 4). The number was significantly larger in group 2 than in group 1 (p = 0.043). VEGF expression, vascular density and macrophage infiltration were not significantly different between groups 2 and 3. There were significant correlations between immunoreactivity to VEGF and the number of CD31-positive vessels (r = 0.824, p < 0.001, Spearman correlation) and the number of CD68-positive macrophages (r = 0.485, p = 0.035).
We observed that immunoreactivity to VEGF was significantly stronger in membranes from active PDR compared to those from inactive PDR. The number of CD31-expressing blood vessels was also significantly higher in the active group. The results are consistent with previous reports that have demonstrated increased VEGF levels in both vitreous humor and serum, and highly expressed VEGF in the endothelial cells of fibrovascular membranes from eyes of PDR patients.1-5 There was a significant correlation between the immunoreactivity to VEGF and the number of CD31-positive vessels, supporting the theory that VEGF may play an important role in the progression of neovascularization in PDR.1,2
In 40% of the eyes that were treated with intravitreal bevacizumab injection and PPV, significant decreases in VEGF expression and vascular densities were observed. In those eyes, the decrease in immunoreactivity to VEGF and vascular densities correlated with the regression of NVD and large caliber vessels and the presence of fibroglial tissue comprising the NVE. However, relatively strong expression of VEGF and high vascular densities were observed in the other 60% of membranes, even after preoperative adjunct bevacizumab injection. In contrast with our observations, previous studies have shown a significant reduction in VEGF level in both the vitreous and aqueous humor in active PDR patients after a single intravitreal bevacizumab injection.16-18 However, these studies reported levels of free VEGF in the vitreous or aqueous humor, unlike the tissue-expressed level as measured in the current study. Kubota, et al.19,20 also demonstrated that vascular endothelial cells were still present in the fibrovascular membranes from PDR patients and trabecular meshwork of neovascular glaucoma even after the intravitreal bevacizumab injection. Based on the observations of the current study and a recent report by Kubota, et al.20 vascular endothelial cells would not disappear in all patients in a short period after the injection of bevacizumab. On the contrary, they showed significant decreases in VEGF expression in fibrovascular membranes after a bevacizumab injection, which is contradictory to our results.20 The tissue-expressed VEGF level may vary greatly among PDR patients, and a single bevacizumab injection would result in a different level of VEGF expression. Further studies of larger groups are warranted to verify these findings. Some reports also demonstrated a clinically favorable outcome associated with intravitreal bevacizumab in active PDR patients. However, even in those reports, partial regression of the active PDR was observed in 34 to 41% of the patients, indicating that a single bevacizumab injection may not be enough to cause complete regression of active PDR.13,21
VEGF is also a chemotactic factor for inflammatory cells, activating monocytes and macrophages, and induces the expression of ICAM-1 and E-selectin on vascular endothelial cells to facilitate adhesion and migration of inflammatory cells.22-27 Since angiogenesis and inflammation are closely related, we hypothesized that VEGF inhibition by bevacizumab may influence new vessel formation as well as inflammatory activity in PDR membranes. In specimens treated with bevacizumab, the number of CD68-positive macrophages was comparable to that of active PDR cases without preoperative adjunctive therapy, and significantly higher than numbers observed in the inactive group. This may be a consequence of bevacizumab's actions to increase inflammation in the process of new vessel regression. Inhibition of VEGF has been shown to induce vascular regression through endothelial cell apoptosis in neovascular vessels.28-30 Macrophages recognize and phagocytose apoptotic cells, and their depletion results in endothelial cell survival and persistence of functional capillaries.31-33 During vascular regression, macrophages may be recruited in the process of endothelial cell apoptosis. Tatar, et al.34 also reported an unexpected increase in inflammatory activity in human choroidal neovascular tissues after intravitreal bevacizumab. Our data corroborate this finding and further indicate that intravitreal bevacizumab injection may stimulate an inflammatory cellular reaction shortly after the drug application.
Our sample population is small and the results are limited. The results of this study might also be influenced by a selection bias because the membranes were obtained from the PDR patients without randomization. Further investigations involving a larger group with appropriate randomization and a control group will be necessary to confirm our preliminary findings. In addition, the effects of intravitreal bevacizumab on VEGF expression were studied only in the fibrovascular membranes in this study. Since intravitreal bevacizumab injections may also affect VEGF expression in the neurosensory retina, including retinal vasculature, and induce possible vascular regression and ischemia as shown in previous case reports,35,36 further studies should include the effect of bevacizumab on the neurosensory retina, especially with vascular perfusion.
In summary, we observed that intravitreal bevacizumab injections cause a significant reduction in VEGF expression and vascular density in a limited number of patients. A single intravitreal bevacizumab injection may be insufficient to induce a complete blockage of VEGF on fibrovascular membranes in active PDR patients. Repeated injections, panretinal photocoagulation and/or PPV may be necessary following intravitreal bevacizumab in order to reinforce its anti-VEGF effect and result in complete regression of active PDR to an inactive membrane.
Figures and Tables
Fig. 1
Representative fundus photographs and histopathologic findings. (A) Group 1. A fibrotic fibrovascular membrane can be seen in this fundus photo. This section of excised tissue shows sparsely vascularized fibrovascular tissue in H&E staining. (B) Group 2 with regression of active PDR. Regressed NVD, large caliber vessels and gliosis are evident. Sparsely vascularized tissue is present in H&E staining. (C) Group 2 with active PDR. (D) Group 3. Proliferative fibrovascular membranes and preretinal hemorrhage can be seen. H&E staining shows highly vascularized tissue (original magnification × 400). PDR, proliferative diabetic retinopathy; NVD, neovascular at the disc.
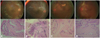
Fig. 2
Immunohistochemistry for vascular endothelial growth factor (VEGF) expression. (A) Group 1. Weak immunoreactivity to VEGF is observed in fibrovascular tissue. (B) Group 2 with regression of active proliferative diabetic retinopathy (PDR). The immunoreactivity to VEGF shows intermediate staining in vascular endothelial cells surrounding the vascular lumen. (C) Group 2 with active PDR (D) Group 3. Strong immunoreactivity to VEGF is shown (original magnification × 400).

Fig. 3
Immunohistochemistry for CD31. (A) Group 1. CD31-positive blood vessels are barely visible in fibrovascular tissue. (B) Group 2 with regression of active PDR. Vascular lumen with CD31-positive endothelial cells is visible. (C) Group 2 with active PDR. (D) Group 3. Highly vascularized tissue with the vascular lumen surrounded by CD31-positive cells (original magnification × 400).

Fig. 4
Immunohistochemistry for CD68. (A) Group 1. CD68-positive cells are barely visible in fibrovascular tissue. (B) Group 2. A significant increase in CD68-positive cells can be seen when compared with (A). (C) Group 3. Moderate increase in CD68-positive cells can be seen (original magnification × 400).

ACKNOWLEDGEMENTS
This work was supported by the National Health Insurance Corporation Ilsan Hospital grant (2009-31).
References
1. Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994. 118:445–450.

2. Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.

3. Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998. 82:561–568.

4. Chen YS, Hackett SF, Schoenfeld CL, Vinores MA, Vinores SA, Campochiaro PA. Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br J Ophthalmol. 1997. 81:919–926.

5. Matsuoka M, Ogata N, Minamino K, Matsumura M. Expression of pigment epithelium-derived factor and vascular endothelial growth factor in fibrovascular membranes from patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2006. 50:116–120.
6. Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005. 25:111–118.
7. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.

9. Meleth AD, Agrón E, Chan CC, Reed GF, Arora K, Byrnes G, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005. 46:4295–4301.

10. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005. 115:1111–1119.

11. Minnella AM, Savastano CM, Ziccardi L, Scupola A, Falsini B, Balestrazzi E. Intravitreal bevacizumab (Avastin) in proliferative diabetic retinopathy. Acta Ophthalmol. 2008. 86:683–687.

12. Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008. 246:1699–1705.

13. Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113:1695.e1–1695.e15.

14. Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009. 116:927–938.

15. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006. 26:699–700.

16. Arimura N, Otsuka H, Yamakiri K, Sonoda Y, Nakao S, Noda Y, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology. 2009. 116:921–926.

17. Matsuyama K, Ogata N, Jo N, Shima C, Matsuoka M, Matsumura M. Levels of vascular endothelial growth factor and pigment epithelium-derived factor in eyes before and after intravitreal injection of bevacizumab. Jpn J Ophthalmol. 2009. 53:243–248.

18. Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007. 125:1363–1366.

19. Kubota T, Aoki R, Harada Y, Tou N, Kohno Y, Tawara A, et al. Trabecular meshwork in neovascular glaucoma eyes after the intravitreal injection of bevacizumab. Br J Ophthalmol. 2009. 93:557–558.

20. Kubota T, Morita H, Tou N, Nitta N, Tawara A, Satoh H, et al. Histology of fibrovascular membranes of proliferative diabetic retinopathy after intravitreal injection of bevacizumab. Retina. 2010. 30:468–472.

21. Arevalo JF, Wu L, Sanchez JG, Maia M, Saravia MJ, Fernandez CF, et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye (Lond). 2009. 23:117–123.

22. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996. 87:3336–3343.

23. Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990. 172:1535–1545.

24. Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002. 160:501–509.

25. Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001. 276:7614–7620.

26. Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S, et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998. 82:845–851.

27. Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994. 55:410–422.

28. Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995. 1:1024–1028.

29. Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006. 290:H547–H559.

30. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006. 290:H560–H576.

31. Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999. 126:2141–2147.

32. Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997. 124:3633–3638.

33. Lang RA. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ. 1997. 4:12–20.

34. Tatar O, Yoeruek E, Szurman P, Bartz-Schmidt KU, Adam A, et al. Tübingen Bevacizumab Study Group. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008. 126:782–790.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download