Abstract
Purpose
A comparison of MRI and computed tomography-myelography (CTM) for lumbar intracanalar dimensions. To compare the capability and reproducibility of MRI and CTM in measuring the cross-sectional morphology of intracanalar lesions of the lumbar spine.
Materials and Methods
MRI and CTM of lumbar disc levels from 61 subjects with various lumbar spinal diseases were studied. Dural area, dural anteroposterior (AP) diameter, dural right-left diameter, and thickness of the ligamentum flavum were measured by two orthopedic surgeons. Each section was graded by degree of stenosis. Absolute value and intra- and inter-observer correlation coefficients (ICC) of these measurements and the associations between MRI and CTM values were determined.
Results
Except for MRI determination of ligament flavum thickness, CTM and MRI and intra- and ICC suggested sufficient reproducibility. When measurements of dural area, dural AP diameter, and RL diameter were compared, values in CTM were significantly (p = 0.01-0.004) larger than those in MRI (CTM/MRI ratios, 119%, 111%, and 105%, respectively). As spinal stenosis became more severe, discrepancies between CTM and MRI in measurements of the dural sac became larger.
Conclusion
Both CTM and MRI provided reproducible measurements of lumbar intracanalar dimensions. However, flavum thickness may be more accurately measured by CTM. Because the differences in the measurements between CTM and MRI are very slight and there is very little data to suggest that the precise degree of stenosis is related to symptoms or treatment outcome, the usefulness of the CTM over MRI needs to be confirmed in future studies.
As society ages, the diagnosis and treatment of degenerative diseases in the lumbar spine become more important. Lumbar spinal canal stenosis (LSCS) is a degenerative condition that results in reduced quality of life in the elderly.1-3 This nervous system syndrome is caused by compression of spinal canal space involving neural tissue of the cauda equina and nerve root, and is characterized by back pain and/or neural symptoms in the lower extremities,4 which makes accurate evaluation of the magnitude of stenosis necessary.
Surgery to improve a patient's quality of life is generally performed after conservative treatment has failed.5 Surgical relief of nerve root and dural tube compression ameliorates symptoms associated with LSCS, including walking tolerance.6,7 Radiological methods are important for diagnosis and precise localization of the stenosis, and also aid in surgical decision-making.8-10 Historically, several methods have been used to image the lumbar spine, including radiography, sagittal tomography, myelography, magnetic resonance imaging (MRI), computed tomography (CT), and CT after myelography (CTM).8,11,12 CTM and/or MRI have become the methods of choice for preoperative radiological evaluation of patients with lumbar spinal stenosis.13
Although MRI is used frequently for the preoperative evaluation of spinal lesions,14,15 it has several drawbacks. For example, the degree of stenosis can be exaggerated on T2-weighted images.9 Although CTM requires lumbar puncture and is thus regarded as invasive,16 this technique is also still in use, and it is unclear as to whether MRI or CTM is better for evaluating lumbar intracanalar dimensions. We have therefore done the following: first, compared absolute values of MRI and CTM in the intracanalar measurement of the lumbar spine; second, compared the reproducibility of MRI and CTM for measuring of intracanalar morphology; and third, evaluated the association of measurements between CTM and MRI, in an attempt to assess whether MRI can replace CTM in preoperative evaluation of patients with LSCS.
We retrospectively studied the MRI and CTM scans performed at our institute between 2005 and 2006 on 189 patients (117 men and 72 women), ranging in age from 46 to 82 years (mean ± SD, 63.5 ± 12.7 years) and diagnosed with LSCS, spondylosis, or lumbar disc herniation. All of them were candidates for surgical treatment of a spinal disorder. Patients with scoliosis, tumors, metastatic tumors, or prior surgery were excluded, for spinal canals with such pathological conditions can be associated with rotation deformity, destruction, and iatrogenic abnormality, which produce bias in the comparison between CTM and MRI. Retrospective use of those images for the present study was approved by the Institutional Review Board.
The MRI scans, performed on a 1.5 T scanner (Signa Twin-speed; GE Healthcare, Fairfield, CT), included sagittal and axial T2-weighted images [sequence type: spine echo, flip angle: 90°, slice thickness: 4 mm, field of view (FOV): 16 cm2, matrix: 288 × 256, reconstructed matrix: 512 × 512 number of excitations (NEX): 3, echo train length: 17, repetition time: 3,600 ms; echo time: 102 ms] from the first lumbar through the first sacral level. In all patients, the lumbar puncture was performed at lower lumbar spine under local anesthesia using a 22 G needle. Then, 10 mL of Iohexol (Omnipaque 240, Daiichi-Sankyo, Tokyo, Japan) was injected intrathecally. Within 2 hours of the intrathecal injection of Iohexol, a CT was performed using a LightSpeed Ultra 16 with Xtream (GE Healthcare, Piscataway, New Jersey, USA) under consistent conditions (rotation time: 0.5 sec, slice thickness: 1.25 mm, beam energy: 120 Kv, FOV: 20 cm2, Matrix: 512 × 512). The radiation dose of CTM was approximately 40 mG in computed tomography dose index (CTDI) vol and 1,400 mGy in dose length product (DLP).
For each patient, sagittal scout scans were taken by MRI and CTM at the midline of the lumbar spine. In order to obtain pairs of MRI and CTM data at exactly the same axial section of each patient's spine, 2 orthopedic surgeons with 8 and 14 years of experience carefully determined that they had the same axial sections. The baseline requirements for CTM and MRI pairs were: 1) the axial section was created by a line connecting the middle point of the anterior and posterior margins of each vertebral body; or 2) the axial section line in the sagittal plane was within 3° of being parallel to the line indicated in 1). Additionally, patients who had all disc levels of L1/2, L2/3, L3/4, L4/5, and L5/S and fulfilled the above requirements were subjected for analyses. Consequently, 61 (305 disc levels, 47 men and 14 women) of the 189 patients satisfied these baseline requirements. Eight levels, however, were excluded, because the CTM images showed no contrast dye due to a complete block of the adjacent levels. Thus, a total of 297 CTM and MRI pairs of the same intervertebral disc levels were analyzed. These included 61 L1/2 levels, 59 L2/3 levels, 59 L3/4 levels, 61 L4/5 levels, and 57 L5/S1 levels.
The grades of LSCS have been defined.17,18 The observers were asked to grade five sequential levels (L1-L2 to L5-S1) on each MRI and CTM by three grades of stenosis: mild, moderate, and severe (Fig. 1). Particular attention was paid to the shape of the dural sac.
No compression of the canal area or reduced size of the canal with compression of the disc (or end plate). The anterior part of the dural canal is convex or straight, and the dural sac is circular or oval in shape (Fig. 1) (upper row).
Reduced size of the canal with compression of the disc (or end plate) and flavum. The anterior part of the dural canal is concave with compression, and shape of the dural sac is triangular or semicircular, or more deformed than grade 1 (Fig. 1) (middle row).
Little cerebrospinal fluid or space identified in the canal (Fig. 1) (lower row).
Inter- and intraobserver agreement for grading of LSCS in MRI and CTM were evaluated, as was agreement of grading by MRI and CTM.
The images were randomized, identified by a code number, and presented independently and in a blind manner to the two observers, who assessed each sequence independently and without access to clinical information. Approximately 1 month elapsed between reviews of the repeated scans. Measurements made included the dural sac, its cross-sectional area (Fig. 2A), the maximum anteroposterior (AP) (Fig. 2B) and right-left (RL) (Fig. 2C) diameters of the dural sac, and the maximum ligamentum flavum thickness. For the latter, the thickness of the ligament flavum at its thickest parts on the right and left were measured, and the average of each was calculated (Fig. 2D).
All measurements were performed using image analysis software (CIS-Image/Viewer for Windows Version 2.6.07; IBM Japan Ltd., Tokyo, Japan) at a resolution of 850 × 1024 pixels. Windowing for MRI and CTM was adjusted in each patient, not in a homogenous manner. Intra- and inter-observer reproducibility of the values obtained via MRI and CTM was evaluated, the absolute values obtained by MRI and CTM were compared, and the ratio of intracanalar quantitative measurements by CTM and MRI was calculated. In addition, the dural area, as determined by MRI and CTM, was compared for each grade of stenosis severity.
Paired t-tests were used to compare absolute values obtained by MRI and CTM. Simple regression analysis, using StatView (SAS Institute, Cary, NC, USA), was performed to assess the relationship between MRI and CTM results. Intra-class correlation coefficients (ICC) of agreement to compare inter- and intraobserver agreement were determined using Systat 8.0 software (SPSS Inc., Chicago, IL, USA). Lumbar spinal canal stenosis (LSCS) grading was evaluated by kappa reliability coefficient analysis with quadratic weighting.18 A 95% confidence interval was used in all statistical tests.
Both MRI and CTM had high degrees of intra-observer ICC (> 0.9) in all measurements (Table 1). Inter-observer ICC was also high (> 0.9), except for measurements of flavum thickness by MRI (0.867) (Table 1). Interobserver ICCs of flavum thickness were higher by CTM (0.913), suggesting that the latter has a higher degree of reproducibility in analyzing ligament flavum. When measurements were sorted by grade of spinal stenosis, the MRI interobserver ICC of the dural area (0.852) and dural AP diameter (0.726) in grade 3 and the CTM interobserver ICC of CTM ligamentum flavum thickness in grade 2 (0.846) were relatively low, as compared with the other grades.
In all subjects, the mean ± S.D. grade of LSCS was 1.9 ± 0.7 by CTM and 1.9 ± 0.7 by MRI; the difference between CTM and MRI was not statistically significant. Intraobserver kappa values of the classification of LSCS for MRI and CTM, respectively, were 0.801 and 0.815 by observer 1 and 0.736 and 0.780 by observer 2. Interobserver kappa values ranged from 0.756 to 0.783 for CTM and from 0.79 to 0.824 for MRI. MRI and CTM classifications of severity corresponded well, and the levels of each classification were similar (Table 2). Cohen's Kappa was 0.824, suggesting a high degree of correspondence.
The measurements for dural area (CTM: 128.1 ± 55.3 mm2, MRI: 109.4 ± 54.8 mm2), dural AP diameter (CTM: 10.4 ± 3.2 mm, MRI: 9.4 ± 2.6 mm), and dural RL diameter (CTM: 15.2 ± 4.8 mm, MRI: 14.4 ± 3.9 mm) were significantly larger on CTM than on MRI (p < 0.0001), by 15% for the dural area, 11% for the dural AP diameter, and by 5% for the dural RL diameter (Fig. 3A, B and C) (Table 3). In contrast, flavum thickness measurements (CTM: 3.4 ± 1.3 mm, MRI: 3.5 ± 1.2 mm) was significantly (p < 0.01) smaller on CTM than MRI (Fig. 3D). Regression analysis between MRI and CTM showed that R2 of the dural area was 0.722 (p < 0.0001), R2 of dural AP diameter was 0.661 (p < 0.0001), R2 of dural RL diameter was 0.682 (p < 0.0001), and R2 of flavum thickness was 0.208 (p < 0.0001).
When patients were sorted according to grade of stenosis, we found that, as stenosis became more severe, the dural area and dural AP and RL diameters were significantly smaller on CTM than on MRI (p < 0.001 each by one-way ANOVA) (Fig. 3A, B and C). For grades 1 and 2, the dural area was significantly larger when measured by CTM than by MRI (Fig. 3A), but there was no significant difference in grade 3. Similarly, for grades 1 and 2, dural AP and RL diameters were significantly greater by CTM than by MRI (Fig. 3B and C). In grades 1 and 3, flavum thickness measurements in CTM were significantly (p < 0.01) smaller than those in MRI (Fig. 3D). Regression analysis of the dural area by stenosis grade between CTM and MRI showed that R2 was 0.741 for grade 1 and 0.631 for grade 2, respectively, indicating a relatively high level of agreement (Fig. 4). In contrast, there was little agreement between the two methods for grade 3 (R2 = 0.155) (Fig. 4). Remarkably, the CTM failed to adequately measure the dural sacs of 10 levels from 10 patients classified as grade 3. These 10 levels, however, could be measured by MRI because the cerebrospinal fluid was reflected (Fig. 5).
Overall, we found that both CTM and MRI were very effective for objective analysis of the shape of the dural sac and the thickness of the ligamentum flavum, and for subjective grading of the severity of spinal stenosis. It is important to note that the dural area was significantly larger when measured by CTM than by MRI. Measurements of the dural area and diameters by each method showed high reproducibility, with good intra- and interobserver ICC, and the two methods yielded comparable ICC values for these measurements. In contrast, the interobserver ICC of the ligamentum flavum was lower on the MRI than on the CTM. CTM may be superior to MRI in distinguishing bone and soft tissue. While the MRI could distinguish the flavum from the dural sac and fat but not from bone, the CTM showed the flavum as a space between the contrasted dural sac and lamina. Comparisons within each stenosis grade showed that, for grade 3, the inter-observer ICCs of the dural area and AP diameter on MRI were relatively low. It is likely that, the more severe the stenosis, the more deformed the shape of the dural sac, and thus dural morphology was less clearly identifiable on the MRI, partly due to susceptibility gradients at bone-soft tissue borders as a well-known source of MRI artifacts. It can also be speculated that the difference in image resolution between the two imaging modalities (MRI: 288 × 256 pixels, CT: 512 × 512 pixels) had a significant impact on these results.
The grade of root compression and LSCS have each been classified into four levels on MRI, with intra- and interobserver reliability kappa scores ranging from 0.57 to 0.76 and from 0.27 to 0.56, respectively.19 In our classification into 3 grades, all intra- and interobserver reliability kappa values were over 0.7 for both CTM and MRI. When classified into these three grades, there were no significant differences in average classification or statistical evaluation between CTM and MRI. These results indicate that CTM and MRI are comparable in classifying the grade of stenosis, and that neither method over- nor underestimates the degree of stenosis.
In agreement with previous results,20 we found that dural area and dural AP and RL diameters in stenosis grades 1 and 2 were significantly larger when measured by CTM than by MRI. There are two possible explanations for these larger measurements of the dural diameters by CTM: First, the brightness of intrathecally injected contrast medium,20,21 can result in larger values from CTM. Second, pulsation of the cerebrospinal fluid and truncation artifact (Gibbs phenomenon)22 can result in smaller values from MRI. However, the precise mechanism for this is not yet clear. We found that the magnification rate (CTM/MRI) of the dural tube area, approximately +15%, is in agreement with the +20% that was previously reported.7 Although the larger visualization of the dural sac by CTM may be a normal radiological phenomenon, clinicians should be aware of this. This may also explain why the flavum thickness, the rest of the space in the spinal canal, was significantly larger by MRI than by CTM.
Comparison of CTM and MRI measurements of the dural sac in grades 1 and 2 stenosis showed high regression conformance, indicating that these two techniques have approximately the same ability to assess dural canal shape in levels of slight to moderate stenosis. However, in grade 3 or severe stenosis, dural shapes shown by MRI and CTM differed considerably. Conformance of ligamentum flavum was very low in all grades of stenosis.
CTM is an invasive procedure with several potential complications, including anaphylactic reactions to the contrast material, headaches, and, rarely, arachnoiditis and infection.23-25 In addition, it involves not small number of doses of radiation9 and is more expensive than MRI. Those issues are indeed important to clinical practice. However, we found that CTM was slightly superior to MRI in the reproducibility of flavum thickness measurements, indicating that CTM is preferable for preoperative planning of lumbar decompression for stenosis. In contrast, stenotic lumbar of level 10 (severe) showed no contrast medium in CTM, while these areas could be measured by MRI. Additionally, CTM cannot measure level 8 due to a complete block of the adjacent levels. Presumably, in the case of severe stenosis, the dye did not pass the block in enough volume to be visible. This is a significant problem in CTM as there may be more that one tight level. Thus, each of these methods has drawbacks, making it necessary to perform the other imaging method for more detailed information.
In conclusion, the following significant results were obtained in this study. The dural cross sectional size is slightly, but significantly, larger when measured by CTM than by MRI. The two methods had equal ability to preoperatively evaluate mild LSCS, but CTM was slightly superior in evaluating ligaments close to bone. For severe stenotic levels, MRI may be more sensitive in evaluating dural size, although its reproducibility was slightly lower than that of CTM. Taking into consideration the invasiveness of CTM, MRI should be the primary screening method of choice. CTM, which is slightly superior to MRI in identifying the bone-soft tissue interface, may be advantageous for patients requiring decompression of neural tissue. However, since there is very little data to suggest that the precise degree of stenosis is related to symptoms or treatment outcome, the small differences found between CTM and MRI cannot directly indicate any clinical relevance.
The design of this study had several limitations. First, we studied lumbar MRI and CTM in a lying-down position, which is commonly used in the clinical practice. However, those images are less clinically relevant than those obtained when standing or under axial loading.26 Second, we compared the results of CTM and MRI scans of the lumbar region in the same patients for a fixed period, so there may have been slight differences in scanning level. It is unclear, however, how this slight difference may have influenced our results. Third, only axial T2-weighted MR images were compared with the CTM scans. Had T1-weighted images been included, a comprehensive analysis of MRI may have shown more obvious differences. Fourth, the difference between stenosis of grades 1 and 2 might not be clinically important. The classification was employed only to evaluate subclinical intracanalar condition of the patients. Fifth, the real values of the intracanalar morphology have not been evaluated, as this was a clinical study of patients. Sixth, the resolutions of the MRI and CTM were slightly different. Windowing for MRI and CT was not standardized. These can potentially create bias in the results of the present study.
Figures and Tables
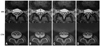 | Fig. 2Measurements of intracanalar morphology using magnetic resonance imaging (MRI, left) and computed tomography-myelography (CTM, right). (A) Dural area: Cross-sectional area of the dural sac. (B) Dural AP diameter: Maximum anteroposterior diameter of the dural sac. (C) Dural RL diameter: Maximum right-left diameter of the dural sac. (D) Ligamentum flavum thickness: Thickness of the ligamentum flavum at its thickest part on the right and left. AP, anteroposterior; RL, right-left. |
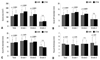 | Fig. 3Average measurements of dural area, dural AP and RL distance, and flavum thickness in each grade of stenosis severity. (A) Dural area: MRI measurements were significantly smaller than CTM measurements for total levels and grades 1 and 2. More severe stenosis was significantly correlated with a smaller cross sectional area of the dural sac in both CTM and MRI (one-factor ANOVA, p < 0.001). (B) Dural AP diameter: MRI measurements were significantly smaller than CTM measurements for total levels and grades 1 and 2. (C) Dural RL diameter: MRI measurements were significantly smaller than CTM measurements for total levels and grades 1 and 2. (D) Flavum thickness: CTM measurements of flavum thickness were significantly smaller than MRI measurements for total levels and grades 1 and 3. AP, anteroposterior; RL, right-left. |
 | Fig. 4Simple regression of dural area in MRI and CTM by stenosis grade: For grades 1 and 2, there were significant correlations between MRI and CTM, with high R2 values (p < 0.01 each). For grade 3, however, R2 was very low. |
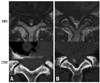 | Fig. 5Typical examples of discrepancies between CTM and MRI in severe stenotic levels. (A) In the MRI, the dural sac could be confirmed. In the CTM, however, dural shape and ligamentum flavum could not be observed. (B) On the MRI, the dural sac is boomerang-shaped with compression by a herniated disc. On the CTM, however, the shape of the dural sac is altered as it becomes partially filled with contrast medium. |
ACKNOWLEDGEMENTS
The authors appreciate a support from Dr. Tomohiro Sasaki (Matsunami General Hospital, Gifu, Japan.) in preparing the images.
References
1. Athiviraham A, Yen D. Is spinal stenosis better treated surgically or nonsurgically? Clin Orthop Relat Res. 2007. 458:90–93.

2. Katz JN, Dalgas M, Stucki G, Katz NP, Bayley J, Fossel AH, et al. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis Rheum. 1995. 38:1236–1241.

3. Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly: an overview. Eur Spine J. 2003. 12:Suppl 2. S170–S175.

4. Miyamoto M, Genbum Y, Ito H. [Diagnosis and treatment of lumbar spinal canal stenosis]. J Nippon Med Sch. 2002. 69:583–587.

5. Gunzburg R, Keller TS, Szpalski M, Vandeputte K, Spratt KF. Clinical and psychofunctional measures of conservative decompression surgery for lumbar spinal stenosis: a prospective cohort study. Eur Spine J. 2003. 12:197–204.
6. Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 6: magnetic resonance imaging and discography for patient selection for lumbar fusion. J Neurosurg Spine. 2005. 2:662–669.

7. Yamazaki K, Yoshida S, Ito T, Toba T, Kato S, Shimamura T. Postoperative outcome of lumbar spinal canal stenosis after fenestration: correlation with changes in intradural and extradural tube on magnetic resonance imaging. J Orthop Surg (Hong Kong). 2002. 10:136–143.

8. Amundsen T, Weber H, Lilleås F, Nordal HJ, Abdelnoor M, Magnaes B. Lumbar spinal stenosis. Clinical and radiologic features. Spine (Phila Pa 1976). 1995. 20:1178–1186.
9. Drew R, Bhandari M, Kulkarni AV, Louw D, Reddy K, Dunlop B. Reliability in grading the severity of lumbar spinal stenosis. J Spinal Disord. 2000. 13:253–258.

10. Onel D, Sari H, Dönmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine (Phila Pa 1976). 1993. 18:291–298.
11. Bolender NF, Schönström NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985. 67:240–246.

12. Gaskill MF, Lukin R, Wiot JG. Lumbar disc disease and stenosis. Radiol Clin North Am. 1991. 29:753–764.
13. Speciale AC, Pietrobon R, Urban CW, Richardson WJ, Helms CA, Major N, et al. Observer variability in assessing lumbar spinal stenosis severity on magnetic resonance imaging and its relation to cross-sectional spinal canal area. Spine (Phila Pa 1976). 2002. 27:1082–1086.

14. Herno A, Airaksinen O, Saari T, Pitkänen M, Manninen H, Suomalainen O. Computed tomography findings 4 years after surgical management of lumbar spinal stenosis. No correlation with clinical outcome. Spine (Phila Pa 1976). 1999. 24:2234–2239.

15. Dorenbeck U, Schreyer AG, Grunwald IQ, Held P, Feuerbach S, Seitz J. Degenerative diseases of the lumbar spine. Comparison of the multiecho data image combination sequence with magnetization transfer saturation pulse versus lumbar myelography/postmyelographic computed tomography. Acta Radiol. 2004. 45:866–873.
16. Sather MD, Gibson MD, Treves JS. Spinal subarachnoid hematoma resulting from lumbar myelography. AJNR Am J Neuroradiol. 2007. 28:220–221.
17. Bartynski WS, Lin L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. AJNR Am J Neuroradiol. 2003. 24:348–360.
18. Rankine JJ, Hutchinson CE, Hughes DG. MRI of lumbar spondylosis: a comparison of sagittal T2 weighted and three sequence examinations. Br J Radiol. 1997. 70:1112–1121.

19. Beattie PF, Meyers SP, Stratford P, Millard RW, Hollenberg GM. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine (Phila Pa 1976). 2000. 25:819–828.

20. Moon ES, Kim HS, Park JO, Shin DE, Ha JW, Shim DJ, et al. Comparison of the predictive value of myelography, computed tomography and MRI on the treadmill test in lumbar spinal steno-sis. Yonsei Med J. 2005. 46:806–811.

21. Burr BA, Resnick D, Syklawer R, Haghighi P. Fluid-fluid levels in a unicameral bone cyst: CT and MR findings. J Comput Assist Tomogr. 1993. 17:134–136.
22. Reul J, Gievers B, Weis J, Thron A. Assessment of the narrow cervical spinal canal: a prospective comparison of MRI, myelography and CT-myelography. Neuroradiology. 1995. 37:187–191.

23. Abla AA, Rothfus WE, Maroon JC, Deeb ZL. Delayed spinal subarachnoid hematoma: a rare complication of C1-C2 cervical myelography. AJNR Am J Neuroradiol. 1986. 7:526–528.
24. Kotilainen E, Sonninen P, Kotilainen P. Spondylodiscitis: an un-usual complication after lumbar myelography. Joint Bone Spine. 2007. 74:113–114.

25. Young DA, Burney RE 2nd. Complication of myelography--tran-section and withdrawal of a nerve filament by the needle. N Engl J Med. 1971. 285:156–157.

26. Willén J, Wessberg PJ, Danielsson B. Surgical results in hidden lumbar spinal stenosis detected by axial loaded computed tomography and magnetic resonance imaging: an outcome study. Spine (Phila Pa 1976). 2008. 33:E109–E115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download