Abstract
Purpose
The objectives of this study are to describe the outcome of adolescent idiopathic scoliosis (AIS) patients treated with Video Assisted Thoracoscopic Surgery (VATS) plus supplementary minimal incision in the lumbar region for thoracic and lumbar deformity correction and fusion.
Materials and Methods
This is a case series of 13 patients treated with VATS plus lumbar mini-open surgery for AIS. A total of 13 patients requiring fusions of both the thoracic and lumbar regions were included in this study: 5 of these patients were classified as Lenke type 1A and 8 as Lenke type 5C. Fusion was performed using VATS up to T12 or L1 vertebral level. Lower levels were accessed via a small mini-incision in the lumbar area to gain access to the lumbar spine via the retroperitoneal space. All patients had a minimum follow-up of 1 year.
Results
The average number of fused vertebrae was 7.1 levels. A significant correction in the Cobb angle was obtained at the final follow-up (p = 0.001). The instrumented segmental angle in the sagittal plane was relatively well-maintained following surgery, albeit with a slight increase. Scoliosis Research Society-22 (SRS-22) scores were noted have significantly improved at the final follow-up (p < 0.05).
Conclusion
Indications for the use of VATS may be extended from patients with localized thoracic scoliosis to those with thoracolumbar scoliosis. By utilizing a supplementary minimal incision in the lumbar region, a satisfactory deformity correction may be accomplished with minimal post-operative scarring.
The prevalence of idiopathic scoliosis with a Cobb angle of more than 10° in adolescents under 16 years of age is 2-3%. Surgical treatment is usually indicated for a patient with a Cobb angle more than 40°, with a predilection for females rather than males (10 : 1).1 In the surgical management of adolescent idiopathic scoliosis (AIS), psychological impact is an important factor to be considered since the patients are usually girls who are concerned about cosmetic and physical appearance, and for them the long postoperative scars may not be acceptable.2 This fact may be a cause of delayed consultation, which may lead to a missed opportunity for appropriate timing of surgical intervention. This may consequently result in progression in the Cobb angle, which further necessitates the need to substantiate the number of fusion levels. It is for this reason that many spine surgeons have tried to concentrate their efforts in exploring minimally invasive access methods to reduce, if not completely eliminate, long and extensive incisions and approaches that would otherwise resort in permanent lifetime disfigurement. Thoracoscope-assisted surgeries have been recently utilized to avoid not only this physical complication, but minimize as well the potential risks in a formal thoracotomy approach. This technique has been presently adapted in the form of Video-Assisted Thoracoscopic Surgery (VATS) for the correction of scoliosis.
In the treatment of thoracic AIS, the posterior spinal fusion technique, which involves segmental instrumentation with either hooks or pedicle screws, is thought to be the gold standard. Several clinical studies have attempted to evaluate the results of the anterior spinal approach compared with posterior spinal instrumentation and fusion. Advantages of an open anterior approach include the ability to fuse a smaller number of vertebral segments, achieve greater correction in coronal plane, and better restoration of kyphosis in thoracic spine, with generally lesser blood loss.3,4 However, anterior surgery via an open thoracotomy approach has some disadvantages, such as pulmonary function impairment, long post-operative scar, and marked post-operative pain. The development of VATS has been an im-portant step for the correction of scoliosis because a compa-rable coronal and sagittal plane correction can be achieved while minimizing the impact on pulmonary function. Decreased post-operative pain and improved cosmetic appearance may be expected as a result of the use of small incisions.5-7 However a major limitation of the VATS technique is that it cannot be used when the distal end of the major curve extends into the lumbar area, as it is not possible to access the retroperitoneal space with a thoracoscope. A possible solution for such cases is to tackle the distal lumbar levels with a mini-open retroperitoneal approach in conjunction with VATS being used for the proximal thoracic part of the curve. Despite these merits, there are no reports about the clinical and radiological outcomes with this operative method in thoracolumbar idiopathic scoliosis. The purpose of our study, therefore, is to show the results of 131scoliosis corrective surgeries done by a combined mini-open retroperitoneal incision and VATS, especially for AIS Lenke classification types 1A and 5C, which necessitate lumbar segment fusion.
We performed spinal fusion with anterior instrumentation in the thoracic spine using VATS combined with a mini-open retroperitoneal approach to the lumbar spine for AIS patients. The results were analyzed retrospectively. There were 5 patients with Lenke type 1A, and 8 patients with Lenke type 5C. Fusion level limits extended from the proximal end vertebra to the distal end vertebra of the main structural curve. The minimum follow-up period was 1 year. We evaluated preoperative as well as post-operative coronal balance, sagittal balance, coronal curve correction, and thoracic kyphosis using preoperative, post-operative, and final follow-up radiographs taken in a standing position. All patients were evaluated using the Scoliosis Research Society-22 (SRS-22) questionnaire which was administered preoperatively and post-operatively. Total scores as well as individual domain scores for pain, self-image, function, mental health, and satisfaction parameters were calculated and analyzed for each patient.
General anesthesia was administered via a double-lumen intubation technique for all patients. The patients were then placed in a lateral position with the arms in the 90/90 position and secured to the table with the help of body tape and restraints to eliminate potential movement of the patient during the surgery. Skin landmark markings for all involved vertebral levels were done with C-arm guidance. Portal sites in line with the anterior and mid-axillary lines were also marked as well as the planned incision markings for the retroperitoneal approach. Two monitors were utilized: The first was positioned in front of and behind the patient to provide better visualization for the surgeons in both sides of the surgical field. Specialized spinal implants (Moss Miami screws, DePuy spine, Rayham, MA, USA) and basic endoscopic instruments were used in the procedure. The instruments included a 0°, 30° scope, harmonic scalpel, long curettes, pituitaries, fan retractor, Kerrison rongeur, silicon ball headed tips, and some standard endoscopic instruments (kitners, endo-bovie, and hemostatic agents). Once the lung was deflated, the proximal portal was first inserted in the mid-axillary line. Upon insertion, digital inspection through the portal was performed to assure the lung was deflated and no adhesions were present. This was followed by the placement of other portals along the mid-axillary as well as anterior axillary line under direct thoracoscopic visualization. Incisions for the portals were placed at 2 interspaces apart, allowing for portal placement above and below the rib at each level, thus enabling the surgeon to reach 2 levels through a single skin incision. At the thoracolumbar junction, an additional portal was needed for the retraction of the diaphragm. After inserting the thoracoscope at the apex, the ribs were counted to confirm the level. The harmonic scalpel was then introduced and the parietal pleura was cut along the longitudinal direction of the spine at all intended levels of instrumentation. The segmental vessels were well-visualized and cauterized using the harmonic scalpel. The pleura was then dissected off anteriorly from the anterior longitudinal ligament and posteriorly from the rib heads. To prevent injury to the contralateral vessels, we inserted a radioopaque-marked gauze into the contralateral space. Discectomy was then performed at all intended levels. A discectomy knife was used to incise the annulus, followed by disc removal in a standard fashion using selected endoscopic curettes, pituitaries, and Kerrison rongeurs. Once the disc was completely removed, the anterior longitudinal ligament was thinned out and then cut from within the disc with a pituitary rongeur. The disc and posterolateral annulus were completely removed. The endplates were rasped until the bleeding subchondral bone was visualized. The disc space was then packed with Surgicel (Ethicon, Somerville, NJ, USA) to control endplate bleeding. Subsequent disks within the intended fusion levels were then prepared in the same fashion. The T12-L1 disc may also be removed from the thoracic cavity depending on the insertion of diaphragm. If the diaphragm inserts below the T12-L1 disc space, then the disc may be removed thoracoscopically, but if the diaphragm inserts above the T12-L1 disc, then the disc must be removed through the retroperitoneal approach. Once all fusion levels were addressed, the scope, instruments, and portals were then removed, and a retroperitoneal approach was performed. The lumbar retroperitoneal incision was performed over the skin markings utilizing the 11th rib approach. If the planned level of instrumentation extends to L2 or L3, then the incision is planned in such a way (usually centering on L2) that it can be used to reach both proximal and distal lumbar levels. For the retroperitoneal approach, a 3-5 cm incision was put over the skin marking to expose the 11th rib. The 11th rib was fully exposed by mobilizing the incision as far anteriorly and posteriorly as possible with careful subperiosteal dissection and subsequent resection using a rib cutter. Deep dissection was then carried through the abdominal muscle layer down to the retroperitoneal space. The peritoneum was then reflected off of the psoas muscle, spine, and diaphragm. Spinal retractors were then inserted. The psoas was retracted posteriorly, and the discs and endplates were exposed and removed in the standard fashion. Once all the discs were removed, iliac bone graft was harvested from the posterior superior iliac spine. The C-arm was then brought into the operating field and positioned over the superior-most vertebral body. A K-wire guide was then placed into the vertebral body just anterior to the rib head. The position was checked with fluoroscopy to ensure that the wire was parallel to the endplates and in the center of the body. A tap was placed over the K-wire and the near cortex was tapped. Care must be taken to prevent K-wire advancement by the tap by holding the outer tip of the exposed K-wire so that it does not advance with each tap rotation. Polyaxial pedicle screws were then inserted with an adequate length to ensure bicortical purchase. Using the rib heads as a reference for subsequent screw placement helps ensure that all screws are in acceptable alignment and proper spinal rotation. The saddles of the screws were then adjusted to line up for introduction of the connecting rod. Pedicle screw heads must be verified that they have similar heights. If a screw is inserted a few millimeters deeper than the rest of the screws, reduction of the rod into the screw head may be difficult. In the lumbar area, 2 screws were inserted in each vertebral body. The first screw was inserted anterior to an imaginary line depicting the posterior 1/3 of the vertebral body and the other was inserted in a converging manner starting near the center of the body and antero-inferior to the first screw. Once all the screws were placed, the surgicel packing was removed from the upper thoracic disc spaces, and the graft material was delivered to the disc space using the graft funnel and plunger. The disc space was completely filled up to the opposite side. The anterior line of lumbar screws was first connected with a short rod. A right angle clamp was then inserted through the retroperitoneal incision and was used to make a small opening under the diaphragm at the center of the vertebral body with the aid of direct thoracoscopic visualization. The opening made should be large enough to permit the passage of the rod. A 5.5-mm rod of appropriate length was then inserted into the chest cavity through the most inferior port, and manipulated superiorly until the rod was completely inside the chest cavity and seated in all saddles of the pedicle screws. It was then manipulated through the small opening made by the right angle clamp and passed into the retroperitoneal space under the diaphragm. The rod was then manipulated into the saddles of the lumbar pedicle screws.
Once the rod was in place, it was sequentially secured to the heads of the screws using screw caps. The proximal thoracic screws were secured first followed by the distal lumbar screws, then finally the middle thoracic screws. The screw caps were then sequentially tightened to produce compression on the convex side and achieve reduction. After all the screw caps were tightened, the saddle extensions of the reduction screws were broken using a customized instrument.
An intercostal drainage tube was then placed through the inferior chest wall portal. All incisions were then closed. Anteroposterior and lateral radiographs were taken and patients were subsequently transferred to the intensive care unit (ICU) for proper postoperative monitoring. We usually hooked up the patient to a ventilator for one night to prevent atelectasis. The patients were then transferred back to the ward the next day. Early mobilization was encouraged on the first or second postoperative day with the aid of a body jacket brace.
A total of thirteen patients (all female) underwent anterior fusion with VATS and mini-open retroperitoneal incision for AIS. According to the Lenke classification, five patients were type 1A and eight patients were type 5C. The mean age at the time of surgery was 13.9 years. All patients had a minimum 12-month follow-up radiograph (range = 12-67 months, mean = 22.5 months). The average number of fused vertebrae was 7.1 (range = 5-9 vertebrae) (Table 1).
The major structural curve was corrected from 43.8° (range = 40-52°) preoperatively to 11° (range = 1-26°) at the time of the latest follow-up. The percentage correction of frontal alignment at the final follow-up was 75% (range = 48-98%). The thoracic kyphosis angle was found to be 16.1° (range = 0-28°) before surgery and 15.5° (range = - 5-31°) at the latest final follow-up after surgery. The lumbar lordosis was 46.6° (range = 20-60°) before surgery and 33.8° (range = 13-62°) after surgery. The average offset of the C7 plumb line (the horizontal distance of a line dropped from the center of the C7 body to the posterior-superior corner of the S1 body) was - 27.3 mm (range = 42-75) before surgery, which improved to - 12.0 mm (range = 92-69) after surgery. The instrumented segmental angle in the sagittal plane was relatively well maintained following surgery, with a slight increase from 8.5° preoperatively to 10.4° post-operatively (Table 2) (Figs. 1 and 2).
The patients-based outcomes as assessed with the SRS-22 questionnaires showed significant improvement in the total score as well as in the self-image domain scores (Table 3).
There was 1 case of intraoperative ureter injury which was diagnosed on the third post-operative day when a relatively large amount of fluid was drained from the retroperitoneal area. A retrograde ureterogram showed injury to the ureter, so a catheter was inserted into the ureter. The catheter was removed after a period of 4 weeks and the patient recovered without any further complication.
One case of delayed pleural effusion was seen. The patient reported dyspnea after removal of the chest tube. A chest PA radiograph taken to investigate showed a moderate pleural effusion. The chest tube was reinserted.
For the management of thoracic and thoracolumbar scoliosis, both posterior spinal fusion and anterior spinal fusion techniques have been cited and compared in various published studies. Anterior surgery was reported to have been able to save one or more fusion levels, no disruption of the posterior extensor musculature (less junctional problem), and afford comparable or improved curve correction with a better derotation ability as compared to posterior surgery for the treatment of thoracolumbar or lumbar scoliosis.8-11 It has also been suggested that anterior correction of deformity is accompanied by superior correction of the compensatory, non-instrumented curve with less post-operative coronal decompensation. On the other hand, the posterior approach with the use of third-generation posterior instrumentation systems has shown a significant improvement over the Harrington distraction rods. Greater control of the sagittal plane was possible with use of third-generation posterior systems which have greater corrective power.11 Due to the ease of extending the fusion cephalad if needed, many authors have recommended using the posterior approach.11 However, a major drawback of posterior surgery is the longer fusion length compared with the anterior approach. The more distal the level of fusion is extended into the lumbar spine, the greater the chances of adjacent disc degeneration in the lower back area, thereby leading to back pain and related problems in early adulthood.12 Conversely, the most important advantage of anterior surgery in the correction of thoracolumbar and lumbar scoliosis is saving a motion segment at the caudal fusion level. Specifically, the important question which needs to be addressed in the management of these types of AIS is whether it is possible to stop at L3 instead of L4, sparing three motion segments instead of two below the fused spine. In several studies, the results have clearly shown that anterior surgery spares at least one motion segment.11,13,14
However, there are several problems associated with anterior instrumentation that have been reported like the increase in kyphosis,11,15,16 pseudoarthrosis,17 and implant failure.11,15,16 An open thoracotomy is also associated with potential complications, such as incisional discomfort, pulmonary dysfunction, pain, and cosmetic disfigurement.
Through continued efforts to improve surgical techniques and safety, thoracoscopic anterior instrumentation and fusion of spine was introduced. Research has demonstrated that thoracoscopic procedures reduce morbidity and at the same time result in reduced length of hospital stay, compared with open thoracotomy procedures.18 Pollock, et al.19 evaluated the efficacy of video-assisted thoracoscopy versus open thoracotomy correction of thoracic scoliosis and found no statistical difference in the correction of the Cobb angle at the time of surgery and 1 year after surgery. Newton, et al.20 likewise evaluated the 2 approaches and found the percent of curve correction for scoliosis to be similar. The authors also demonstrated in an animal model that both thoracoscopic and open anterior release techniques with disc excision results in similar efficacy.21 Picetti et al. noted in several studies that thoracoscopic procedures may be as efficacious as open procedures, as determined in a biomechanical study demonstrating that both surgeries achieve approximately the same degree of curve correction with an average curve correction of 68.6% in endoscopic-treated thoracic scoliosis.3,22
Loner, et al.23 compared video-assisted thoracoscopic spinal fusion with posterior spine fusion with thoracoscopic pedicle screws in thoracic AIS and found that VATS can produce equivalent results to another technique in terms of radiologic, clinical outcomes, and complication rate, if the curves are less than 70 degrees, single thoracic with a normal or hypokyphotic angle.
Five year outcomes after VATS showed comparable results with those of anterior and posterior techniques, and the radiographic findings, pulmonary function, and clinical measures demonstrated no significant difference between the two and five-year follow-up time points also.24
There are, however, limitations and challenges to the VATS technique. These are the inability to endoscopic cross the diaphragm, to maintain retroperitoneal exposure after the diaphragm had been taken down, and attaining lumbar lordosis through structural graft application. Due to these procedural limitations, VATS is difficult to use in the surgery of patients who require fusion at levels extending to the lumbar regions.
In our study, the mini-open retroperitoneal approach in conjunction with VATS enabled us to fuse the curves completely including both the thoracic and lumbar levels. The post-operative correction results of our 13 patients suggest that these techniques are effective in correcting the AIS deformity, with the benefit of being less invasive resulting to less post-operative scarring. The decreased post-operative scarring also seems to affect the patient-based outcomes as assessed with the SRS-22 questionnaire, which revealed a significant improvement in the total scores and in the self-image domain scores. In a comparative study by Lonner, et al. between VATS and posterior instrumentation using a SRS-22 outcome instrument, he noted that VATS patients scored higher in the self-image, mental health, and total domains despite similar curve corrections and hypothesize that this may be related to the smaller surgical scar and less invasive nature of VATS. These results show that for the patients of AIS, cosmetic is an important factor and hence an important determinant of post-operative satisfaction.25
We did not encounter any complications that necessitated revision surgery. There were two minor complications. One was an injury to the ureter incurred by traction during the retroperitoneal approach. This case was easily diagnosed and treated with retrograde ureterogram and cystoscopic catheter insertion. We believe that this iatrogenic complication is an isolated event and could be subsequently prevented in future procedures as the learning curve improves. The other complication was a delayed pleural effusion that was addressed with a simple reinsertion of the chest tube.
The weakness of this study was that it was retrospective in nature with a small population of subjects and no randomization methodology. We recommend that further studies be undertaken with a bigger population in order to allow randomization to facilitate a head-to-head study with posterior instrumentation techniques.
In conclusion, we have presented a novel approach to the thoracolumbar curve by utilizing the current VATS technique and extending its presently accepted indication from patients with localized thoracic scoliosis to those with thoracolumbar scoliosis by the addition of a mini-incision on the lumbar area. By combining the advantages of an anterior approach (short segment fixation) with the VATS technique (cosmetically acceptable scar) and sparing a full diaphragm incision, a satisfactory and significant corrective surgical outcome may be achieved.
Figures and Tables
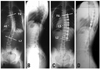 | Fig. 1Preoperative posteroanterior (A) and lateral (B) radiograph of 12 year-old girl with 40O T6-L2 Lenke type 1A curve. The post-operative 2 year follow-up posteroanterior (C) and lateral (D) radiograph of same patient after VATS and lumbar mini-open surgery and fusion. VATS, Video Assisted Thoracoscopic Surgery. |
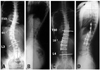 | Fig. 2Preoperative posteroanterior (A) and lateral (B) radiograph of 14 year-old girl with 44O T10-L3 Lenke type 5C curve. The post-operative 3 year follow-up posteroanterior (C) and lateral (D) radiograph of same patient after VATS and lumbar mini-open surgery and fusion. VATS, Video Assisted Thoracoscopic Surgery. |
References
1. Weinstein SL. Adolescent idiopathic scoliosis: prevalence and natural history. Instr Course Lect. 1989. 38:115–128.
2. Noonan KJ, Dolan LA, Jacobson WC, Weinstein SL. Long-term psychosocial characteristics of patients treated for idiopathic scoliosis. J Pediatr Orthop. 1997. 17:712–717.

3. Picetti GD 3rd, Ertl JP, Bueff HU. Anterior endoscopic correction of scoliosis. Orthop Clin North Am. 2002. 33:421–429.

4. Bernstein RM, Hall JE. Solid rod short segment anterior fusion in thoracolumbar scoliosis. J Pediatr Orthop B. 1998. 7:124–131.

5. Landreneau RJ, Hazelrigg SR, Mack MJ, Dowling RD, Burke D, Gavlick J, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993. 56:1285–1289.
6. Newton PO, Marks M, Faro F, Betz R, Clements D, Haher T, et al. Use of video-assisted thoracoscopic surgery to reduce perioperative morbidity in scoliosis surgery. Spine (Phila Pa 1976). 2003. 28:S249–S254.

7. Kim HS, Park JO, Nanda A, Kho PA, Kim JY, Lee HM, et al. Video-assisted thoracoscopic surgery for correction of adolescent idiopatic scoliosis: comparison of 4.5 mm versus 5.5 mm rod constructs. Yonsei Med J. 2010. 51:753–760.

8. Brodner W, Mun Yue W, Möller HB, Hendricks KJ, Burd TA, Gaines RW. Short segment bone-on-bone instrumentation for single curve idiopathic scoliosis. Spine (Phila Pa 1976). 2003. 28:S224–S233.

9. Betz RR, Shufflebarger H. Anterior versus posterior instrumentation for the correction of thoracic idiopathic scoliosis. Spine (Phila Pa 1976). 2001. 26:1095–1100.

10. Burton DC, Asher MA, Lai SM. Patient-based outcomes analysis of patients with single torsion thoracolumbar-lumbar scoliosis treated with anterior or posterior instrumentation: an average 5- to 9-year follow-up study. Spine (Phila Pa 1976). 2002. 27:2363–2367.

11. Suk SI, Lee CK, Chung SS. Comparison of Zielke ventral derotation system and Cotrel-Dubousset instrumentation in the treatment of idiopathic lumbar and thoracolumbar scoliosis. Spine (Phila Pa 1976). 1994. 19:419–429.
12. Cochran T, Irstam L, Nachemson A. Long-term anatomic and functional changes in patients with adolescent idiopathic scoliosis treated by Harrington rod fusion. Spine (Phila Pa 1976). 1983. 8:576–584.

13. Hee HT, Yu ZR, Wong HK. Comparison of segmental pedicle screw instrumentation versus anterior instrumentation in adolescent idiopathic thoracolumbar and lumbar scoliosis. Spine (Phila Pa 1976). 2007. 32:1533–1542.

14. Luk KD, Leong JC, Reyes L, Hsu LC. The comparative results of treatment in idiopathic thoracolumbar and lumbar scoliosis using the Harrington, Dwyer, and Zielke instrumentation. Spine (Phila Pa 1976). 1989. 14:275–280.

15. Lowe TG, Peters JD. Anterior spinal fusion with Zielke instrumentation for idiopathic scoliosis. A frontal and sagittal curve analysis in 36 patients. Spine (Phila Pa 1976). 1993. 18:423–426.

16. Moskowitz A, Trommanhauser S. Surgical and clinical results of scoliosis surgery using Zielke instrumentation. Spine (Phila Pa 1976). 1993. 18:2444–2451.

17. Kostuik JP, Carl A, Ferron S. Anterior Zielke instrumentation for spinal deformity in adults. J Bone Joint Surg Am. 1989. 71:898–912.

18. Landreneau RJ, Hazelrigg SR, Mack MJ, Dowling RD, Burke D, Gavlick J, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993. 56:1285–1289.

19. Pollock ME, O'Neal K, Picetti G, Blackman R. Results of video-assisted exposure of the anterior thoracic spine in idiopathic scoliosis. Ann Thorac Surg. 1996. 62:818–823.

20. Newton PO, Wenger DR, Mubarak SJ, Meyer RS. Anterior release and fusion in pediatric spinal deformity. A comparison of early outcome and cost of thoracoscopic and open thoracotomy approaches. Spine (Phila Pa 1976). 1997. 22:1398–1406.
21. Newton PO, Cardelia JM, Farnsworth CL, Baker KJ, Bronson DG. A biomechanical comparison of open and thoracoscopic anterior spinal release in a goat model. Spine (Phila Pa 1976). 1998. 23:530–535.

22. Picetti GD 3rd, Pang D, Bueff HU. Thoracoscopic techniques for the treatment of scoliosis: early results in procedure development. Neurosurgery. 2002. 51:978–984.

23. Lonner BS, Auerbach JD, Estreicher M, Milby AH, Kean KE. Video-assisted thoracoscopic spinal fusion compared with posterior spinal fusion with thoracic pedicle screws for thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2009. 91:398–408.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download