Abstract
High-dose methotrexate-based chemotherapy has extended survival in patients with primary central nervous system lymphoma (PCNSL). However, although salvage treatment is necessary in recurrent and refractory PCNSL, this has not been standardized. We herein describe the efficacy of a combination of rituximab and temozolomide (TMZ) in two consecutive patients with recurrent and refractory PCNSL. Based on the immunohistochemical study, case 1 had a non-germinal center B-cell-like (non-GCB) subtype, was positive for bcl-2 and negative for O6-methylguanine-DNA methyltransferase (MGMT). Case 2 was GCB subtype, bcl-2-, and MGMT+. Because of the positive expression of MGMT, interferon-beta was additionally given in case 2. Complete responses and partial responses were obtained after the third and fourth cycles of combination therapy, respectively. This was maintained for 12 months, with acceptable toxicity. The combination of rituximab and TMZ was effective in tumors with different immunohistochemical profiles. This combination therapy warrants further study in a larger population.
The use of high-dose methotrexate (MTX)-based systemic chemotherapy has improved the survival of patients with primary central nervous system lymphoma (PCNSL).1 However, salvage treatment is necessary in patients with recurrent and refractory disease. The optimal salvage therapy is not yet known.
Temozolomide (TMZ) is an alkylating agent, and has emerged as a treatment option for PCNSL because of its good penetration of the blood-brain barrier (BBB) and mild toxicity profile. Rituximab is a chimeric monoclonal antibody that targets the B cell specific CD-20 antigen. Synergism has been observed for the combination of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in patients with systemic, CD20-positive diffuse large B-cell lymphoma (DLBCL).2 Such synergism allows for a combination of rituximab and TMZ in the treatment of PCNSL.
We describe our experience using a combination of rituximab and TMZ in two consecutive patients with recurrent and refractory PCNSL.
A 53-year-old man was admitted to our hospital in August 2007 with gait deterioration and urinary incontinence despite absence of meningeal dissemination. He was previously diagnosed as having a PCNSL in the left basal ganglia and the left temporal lobe in 2005, and the pathological diagnosis was confirmed as DLBCL, having a non-germinal center B-cell-like (non-GCB) subtype (CD10-/bcl-6-/MUM1+); immunopositive for CD20; positive for bcl-2 and negative for O6-methylguanine-DNA methyltransferase (MGMT), by immunohistochemical studies. After treatment with intravenous infusion of high-dose MTX (3.5 mg/m2, 1032every 14 days, 3 cycles) followed by radiotherapy (30 Gy whole brain plus a local boost of 10 Gy for the residual tumor), he had been in complete remission (CR) for a total of 20 months. Eastern Cooperative Oncology Group performance status (PS) was listed as 2 due to motor aphasia and right hemiparesis during in CR.
On admission, magnetic resonance image (MRI) revealed a small recurrent tumor in the right frontal lobe (Fig. 1A). PS had deteriorated to 3. The combination of rituximab and TMZ was started (TMZ 150 mg/m2 on day 1 to 5 and intravenous rituximab 375 mg/m2 on day 6, every 28 days). After 3 cycles of this combination chemotherapy, MRI revealed CR of the tumor (Fig. 1B). Thereafter, he received a total of 8 cycles of maintenance TMZ monotherapy; 150 mg/m2 for the first 5 days, escalated to 200 mg/m2 in following cycles, every 28 days. No corticosteroids were given during the combination chemotherapy and TMZ monotherapy. He experienced no toxicity during the combination chemotherapy, although grade 3 leukopenia was observed during the TMZ monotherapy. The last patient follow-up was in November 2008. Although PS had deteriorated to 4 due to motor aphasia, right hemiparesis, incontinence, and cognitive dysfunction, he has remained in CR for 12 months.
A 66-year-old man was admitted to our hospital in March 2007 with right hemiparesis. MRI revealed a large tumor in the left basal ganglia and left temporal lobe (Fig. 2A). Partial resection of the tumor was performed and the diagnosis was DLBCL, having a GCB subtype (CD10+/bcl-6+/MUM1+); immunopositive for CD20; negative for bcl-2 and positive for MGMT. Three cycles of intravenous high-dose MTX resulted in disease progression on MRI. He then received radiotherapy, achieving a partial response (Fig. 2B). PS was listed as 4 because of motor aphasia, right hemiplegia, incontinence, and cognitive dysfunction. The combination of rituximab and TMZ was initiated in August 2007. Because of the expression of MGMT, 3×108 international units interferon-beta was given intravenously before administration of TMZ on day 1. After 4 cycles of this combination chemotherapy, MRI revealed further tumor shrinkage (Fig. 2C). This was followed by 8 cycles of maintenance TMZ monotherapy; 150 mg/m2 for 5 days, every 28 days. This resulted in further tumor shrinkage on MRI, and PS improved from 4 to 3. During the combination chemotherapy and TMZ monotherapy, corticosteroids were not given, and no significant side effects were observed. Because an early relapse was anticipated from his clinical course, combination chemotherapy of rituximab, TMZ, and interferon-beta was administered every 28 days, after TMZ monotherapy to suppress radiologically undetectable recurrence. However, after 4 cycles of this combination chemotherapy, MRI showed multiple tumor recurrences (Fig. 2D). The response duration in this patient was 12 months.
A phase II trial of TMZ monotherapy (150 mg/m2, 5 days in a 28-day cycle) in patients with recurrent and refractory PCNSL reported a 31% objective response rate, and a median overall survival of 3.9 months.3 Although TMZ is an active chemotherapeutic agent for salvage therapy, not all patients benefit and its efficacy is limited. One of the mechanisms leading to TMZ resistance is the production of a DNA repair enzyme MGMT.4 Interferon-beta reportedly lowers MGMT activity in malignant gliomas and enhances the effect of TMZ in vivo and in vitro studies.5-7 Interferon might not cross the BBB, but it also has various direct or indirect biological effects on glioma cells, such as antiangiogenic, immunomodulatory, cell cycle inhibitory, and apoptotic effects.8,9 The cause of the recurrence in case 2 may be speculative, and there may be a possibility that lymphoma islets secluded behind an intact BBB were not reached by interferon and rituximab.
In systemic DLBCL, adverse prognostic influence of non-GCB subtypes, negative bcl-6 expression, and positive expression of bcl-2 has been reported.10-12 The addition of rituximab to CHOP therapy has improved survival for both GCB and non-GCB subtypes, and the status of bcl-6 and bcl-2 appears to have no influence on the outcome, implying that the addition of rituximab overcomes the adverse prognostic influence of negative bcl-6 expression and positive expression of bcl-2 on survival in systemic DLBCL.10-12 Most PCNSL tumors were classified as non-GCB subtype, and the prognostic impact of bcl-6 and bcl-2 status remains controversial.13-15
Two retrospective series of a combination of rituximab and TMZ as salvage chemotherapy against PCNSL have been reported.16,17 One study with 15 patients reported a 53% objective response rate and a median overall survival of 14 months with a 7 days on/7 days off schedule of TMZ combined with rituximab in a 28-day cycle, followed by maintenance treatment with TMZ.16 In the other study, 7 patients received 5 days of TMZ with rituximab in a 28-day cycle, followed by maintenance monotherapy of TMZ.17 That study reported a 100% objective response rate and a median overall survival of 8 months. The prognostic influence of tumor MGMT status, as well as that of subtype bcl-2 and bcl-6 expression, in patients receiving rituximab plus TMZ remains to be examined.
In the present two consecutive cases, although both had a poor PS and the immunohistochemical profiles differed; rituximab/TMZ/±interferon-beta combination treatment was effective. Despite limited data, the observed responses are encouraging and warrant further investigation in a larger population.
Figures and Tables
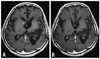 | Fig. 1Gd-enhanced T1-weighted MRI of case 1: (A) MRI on admission in August 2007, showing the recurrent tumor in the right caudate head. (B) MRI after 3 cycles of combination therapy. The recurrent tumor in the right caudate head has disappeared. MRI, magnetic resonance image. |
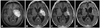 | Fig. 2Gd-enhanced T1-weighted MRI of case 2: (A) Initial MRI in March 2007 shows a large tumor in the left basal ganglia and temporal lobe. (B) MRI after treatment with high-dose MTX chemotherapy and radiotherapy shows partial response of the tumor. (C) MRI after 4 cycles of combination therapy. The tumor has reduced in size. (D) MRI in November 2008. Although the tumor in the left basal ganglia shows further shrinkage, multiple new lesions are observed. MRI, magnetic resonance image; MTX, methotrexate. |
References
1. Pels H, Schlegel U. Primary central nervous system lymphoma. Curr Treat Options Neurol. 2006. 8:346–357.

2. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:235–242.

3. Reni M, Zaja F, Mason W, Perry J, Mazza E, Spina M, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007. 96:864–867.

4. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005. 352:997–1003.

5. Fujimaki T, Ishii H, Matsuno A, Arai H, Nakagomi T. Effectiveness of interferon-beta and temozolomide combination therapy against temozolomide-refractory recurrent anaplastic astrocytoma. World J Surg Oncol. 2007. 5:89.

6. Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005. 65:7573–7579.

7. Park JA, Joe YA, Kim TG, Hong YK. Potentiation of antiglioma effect with combined temozolomide and interferon-beta. Oncol Rep. 2006. 16:1253–1260.
8. Wiranowska M, Wilson TC, Thompson K, Prockop LD. Cerebral interferon entry in mice after osmotic alteration of blood-brain barrier. J Interferon Res. 1989. 9:353–362.
9. Matsuno A, Fujimaki T, Mizutani A, Ide F, Tanaka H, Asano S, et al. Disappearance of gadolinium enhancement in a chemoresistant astrocytoma of the tectum after high-dose interferon beta. Tumori. 2008. 94:853–855.

10. Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Sebban C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003. 101:4279–4284.
11. Winter JN, Weller EA, Horning SJ, Krajewska M, Variakojis D, Habermann TM, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006. 107:4207–4213.

12. Fu K, Weisenburger DD, Choi WW, Perry KD, Smith LM, Shi X, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008. 26:4587–4594.

13. Camilleri-Broët S, Crinière E, Broët P, Delwail V, Mokhtari K, Moreau A, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006. 107:190–196.

14. Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006. 12:1152–1156.

15. Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003. 9:1063–1069.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download