Abstract
Weight-control drugs (known as anorexigens) such as fenfluramine have been linked with pulmonary hypertension in previous reports. In our case, a 29 year old woman was admitted for shortness of breath and was diagnosed with pulmonary hypertension. Three months ago, she had been taking phentermine for five weeks. Other factors that might have contributed to the development of pulmonary hypertension were excluded. With treatment, her symptoms improved. This is the first case that can suggest a possible connection between phenermine single medication with pulmonary hypertension. Phentermine has been considered a relatively safe drug to treat obesity, and further investigation is needed to decide the safety and dosage of phentermine.
Obesity is a serious issue that no longer remains a simple problem of aesthetics but signifies a crisis of health. Evidence shows strong connections between mortality from chronic diseases and obesity1,2 that has led to classifying obesity as a treatable disease. One such treatment, appetite-suppressants, known as anorexigens, has been developed and prescribed to patients for many years. However, some of these medications have had a history of harmful side effects with unacceptable mortality and morbidities.3,4
In Europe during 1960s, the after-effects of aminorex has been grossly tied with pulmonary arterial hypertension (PAH). Also, in 1980s there were reports of primary pulmonary hypertension (PPH) and valvulopathy in patients who were treated with fenfluramine.5-9 As a compound, phentermine and fenfluramine have similar structures (Fig. 1), and both compounds have often been prescribed concurrently as an appetite-suppressant when in the 1980s their concurrent usage was reported to have a synergistic effect.10 In time, fenfluramine-phentermine (Fen-Phen) concurrent medication was proven to play a certain role in the development of PPH and valvular heart diseases. However, there has been no reported study that made a case against phentermine single medication to date.
In this case, we report that phentermine single medication causes the same effects of increased prevalence of PAH as those of single fenfluramine or Fen-Phen concurrent medication.
Three month before hospitalization, a 29 year-old woman sought medical help for obesity. She was otherwise healthy and did not smoke cigarettes. There was no family history of pulmonary hypertension. She had not been pregnant and did not take any other medications including oral contraceptives and herbal medications at the time of symptom onset. She weighted 73 kg and was 1.60 m (65 in.) tall. Her body-mass index (the weight in kilograms divided by the square of the height in meters) was 28. Oral medication therapy with phentermine HCl (adipex®) at a dose of 37.5 mg taken every morning was prescribed. The medication continued for 35 days, during which the patient lost 13 kg. After two months since the start of the prescription, the patient began to feel tachycardia and she was admitted for shortness of breath. Laboratory investigation did not show abnormal findings. Chest radiographs showed mild prominence of the right ventricular outflow tract. Right ventricular hypertrophy was shown in electrocardiography (Fig. 2) while echocardiography showed moderate pulmonary hypertension (right ventricular pressure: 63 mmHg) with tricuspid regurgitation and D-shaped left ventricle (LV) (Fig. 3). There was no pericardial effusion. Cardiac catheterization showed moderate pulmonary hypertension and elevated pulmonary vascular resistance. The right ventricular pressure was 70/29 mmHg; the pulmonary-artery pressure was 69/26 mmHg, with a mean of 42 mmHg while resting; the pulmonary-capillary wedge pressure was 7 to 11 mmHg. A heart MRI showed no visible intracardiac shunt or pulmonary thromboembolism. Other studies were performed to rule out various causes of pulmonary hypertension. Liver-function studies showed a mild elevation of alanine aminotransferase. Abdominal ultrasonography revealed no liver abnormalities. Tests for anti-centromere antibodies and anti-HIV Ag were negative.
Bosentan 125 mg was prescribed with diuretics. Radical anticoagulation therapy was administered. The patient was discharged after three days and followed-up with subjective symptom improvement.
Phentermine is an anorexigen which had been approved for short-term use as a treatment of obesity by US FDA in 1959, and has been used since. Phentermine stimulates the secretion of noradrenalin in the central nervous system, and suppresses appetite by regulatingthe β-adrenergic receptors.11 When phentermine and fenfluramine combination treatment was reported to have a synergistic effect on the dopamine and serotonin release in the rat brain,10 Fen-Phen treatment became the mainstay of anorexin treatment of the 1980s. However, this combination treatment was suggested to have strong ties with PAH and valvular heart disease while fenfluramine single medication was also reported with the same side-effects that in November 1997 the US FDA banned the prescription of fenfluramine altogether. However, to date, the FDA still allows the short term use of phentermine. In the 2000 report by Rich, et al.,12 concerning the surveillance data on anorexigens and pulmonary hypertension, the only drug that had a meaningfully causal relationship with PPH amongst antidepressants, anorexigens, and amphetamines was fenfluramines. So far, there exists very few cases and no controlled studies suggesting a connection between PPH and the use of other appetite suppressants such as phentermine.13,14
A concrete evaluation on the safety of phentermine single treatment is still unavailable and there has not been a reported case since 2000.15 However, there had been studies in the 1990s that raised suspicions that phentermine might have similar correlations with PAH as with fenfluramine. In one study, fenfluramine-like medications were suggested to increase the risk of PPH via affecting serotonin [5-Hydroxytryptamine (5-HT)] transporters.16
Rothman, et al.17 reported that some medications known to increase the risk of PPH (e.g., aminorex, fenfluramine, and chlorphentermine) are 5-HT transporter substrates. The role of 5-HT transporters in the development of PAH has been well-evaluated. 5-HT transporter substrates are translocated into pulmonary cells where, depending on the degree of drug retention, their intrinsic toxicity, and individual susceptibility, PPH may develop as a response to concentrations of these drugs or metabolites. According to Rothman, the duration and dosage of the drug also had a direct relationship with the increase in the relative risk of PAH after treatment. If this evidence can be supported by a considerable number of clinical cases, the FDA must amend its decision to allow the usage of a drug that is now in use without safe guidelines. The current Physician's Desk Reference (PDR) guideline allows phentermine for a duration of 3-6 weeks; however, in our case, the patient had developed PAH with 5 weeks of medication.
In summary, we propose that the usage of phentermine might be associated with PAH by presenting the above case. Although phentermine has been considered a safe drug for obesity, further study should be underway to establish safety and dosage of phentermine.
Figures and Tables
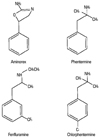 | Fig. 1Chemical structure of anorexigens. Note structural similarity of phentermine and fenfluramine. |
References
1. Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007. 298:2028–2037.

2. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999. 341:1097–1105.

3. Mark EJ, Patalas ED, Chang HT, Evans RJ, Kessler SC. Fatal pulmonary hypertension associated with short-term use of fenfluramine and phentermine. N Engl J Med. 1997. 337:602–606.

4. Dillon KA, Putnam KG, Avorn JL. Death from irreversible pulmonary hypertension associated with short-term use of fenfluramine and phentermine. JAMA. 1997. 278:1320.

5. Schembre DB, Boynton KK. Appetite-suppressant drugs and primary pulmonary hypertension. N Engl J Med. 1997. 336:510–511. author reply 512-3.
6. Goldstein SE, Levy Y, Shoenfeld Y. [Development of pulmonary hypertension and multi-valvular damage caused by appetite depressants]. Harefuah. 1998. 135:489–492. 568
7. Tellier P. Fenfluramines, idiopathic pulmonary primary hypertension and cardiac valve disorders: facts and artifacts. Ann Med Interne (Paris). 2001. 152:429–436.
9. Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. Am Heart J. 2002. 144:1065–1073.

10. Wellman PJ, Maher TJ. Synergistic interactions between fenfluramine and phentermine. Int J Obes Relat Metab Disord. 1999. 23:723–732.

11. Roth JD, Rowland NE. Efficacy of administration of dexfenfluramine and phentermine, alone and in combination, on ingestive behavior and body weight in rats. Psychopharmacology (Berl). 1998. 137:99–106.

12. Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest. 2000. 117:870–874.
13. Schnabel KH, Schulz V, Busch S, Just H. [Drug-induced primary vascular pulmonary hypertension. Contribution to its etiology and clinical course]. Med Welt. 1976. 27:1300–1303.
14. Backmann R, Dengler H, Gahl K, Greiser E, Jesdinsky HJ, Loogen F. [Primary pulmonary hypertension. Report of the Commission of the German Society for Research on Blood Circulation]. Verh Dtsch Ges Kreislaufforsch. 1972. 38:134–141.
15. Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002. 26:262–273.

16. Eddahibi S, Adnot S. Anorexigen-induced pulmonary hypertension and the serotonin (5-HT) hypothesis: lessons for the future in pathogenesis. Respir Res. 2002. 3:9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download