Abstract
Purpose
In treating schwannoma patients, it is critical to determine the origin of the tumor to preserve nerve function. We evaluated the validity of preoperative imaging studies in distinguishing the neurological origin of the schwannomas of the head and neck, and the efficacy of intracapsular enucleation in preserving nerve function.
Materials and Methods
In 7 cases of schwannomas in the head and neck region, we predicted whether the tumor originated from the vagus nerve or the cervical sympathetic chain through imaging studies including computed tomography (CT) and magnetic resonance imaging (MRI). All patients were performed intracapsular enucleation, and the function of the vagus nerve and the sympathetic nerve was evaluated preoperatively and postoperatively.
Results
Preoperative imaging studies showed 6 cases where the tumor was located between the carotid artery and the internal jugular vein, and 1 case where the tumor was located posteriorly, displacing the carotid artery and the internal jugular vein anteriorly. At the time of operation, we confirmed schwannoma originating from the vagus nerve on the first 6 cases, and schwannoma originating from the sympathetic nervous system on the last case. All patients went through successful intracapsular enucleation, and of the seven schwannoma cases, 6 patients maintained normal postoperative neurological function (85.7%).
Schwannoma is a benign neural sheath tumor, and it occurs as a single entity in many cases. It also occurs in overall body areas including the head and neck region. As a slowly-growing benign tumor, it has been reported to occur in the head and neck region in approximately 25-40% of total schwannoma cases.1 It involves the cranial nerves such as V, VII, IV, X, XI, and XII or the sympathetic and peripheral nerves.2 Schwannomas surround many kinds of cranial nerves or other nerves could occur in the head and neck region, it is known that most schwannomas occurring in the head and neck region generally originate from the vagus nerve or sympathetic nervous system, and various preoperative image studies are used to distinguish its location and origin.3 The accepted treatment of schwannoma is surgical resection, and several surgical modalities have been introduced to preserve the neurological functions.4-6
Recently, intracapsular enucleation has been introduced for the preservation of the neurological functions. In this study, we evaluated the validity of preoperative imaging studies in distinguishing the neurological origin of the schwannomas of the head and neck, and the efficacy of intracapsular enucleation in preserving the nerve function.
This study was conducted on seven patients who were suspected with schwannoma at the Department of Otorhinolaryngology Gangnam Severance Hospital from March 2003 to September 2009. All the patients complained of a neck mass as a major symptom. Six patients had normal nerve function, the other one complained of ptosis. There were three men and four women, whose ages ranged between 46 and 71. Computed tomography (CT) and magnetic resonance imaging (MRI) were performed to examine the location of the tumor and its correlation with the carotid artery and the internal jugular vein. Whether the tumor displaced the internal jugular vein and carotid artery to the same direction or to the opposite directions was evaluated.
After informed consent, all patients underwent intracapsular enucleation via the transcervical approach under general anesthesia. After exposing the tumor in the carotid sheath, a vertical incision parallel to the direction of the nerve was made on the capsule, after confirming that the nerve fibers surrounded the tumor. Then intracapsular enucleation was performed as the tumor was carefully dissected from the capsule without any damages given to the nerve fibers. Special attention was paid to preserve the lymphovascular structure within the nerve sheath. The function of the vagus nerve and the sympathetic nerve was evaluated preoperatively and postoperatively by examining vocal cord mobility with laryngoscope and symptoms of Hornor's syndrome.
Preoperative imaging studies showed 6 cases where the tumor located between the carotid artery and the internal jugular vein, and 1 case where the tumor located posteriorly displacing the carotid artery and the internal jugular vein anteriorly (Figs. 1 and 2). At the time of operation, we confirmed schwannoma originating from the vagus nerve on the first 6 cases, and schwannoma originating from the sympathetic nervous system on the last case (Table 1). The tumor was surrounded by the nerve fibers in all 7 cases, and an incision was made on the capsule. The tumor was carefully dissected from the capsule while preserving the nerve fibers, and intracapsular enucleation was performed (Figs. 3 and 4). The preoperative and postoperative neurological functions were evaluated. Of the six vagal schwannomas, five cases maintained normal postoperative neurological function. In the case of sympathetic schwannoma, there were no aggravated neurological deficits except for the ptosis which was observed preoperatively (Table 2).
It is well-known that schwannoma occurring in the head and neck region mostly originates from the vagus nerve or sympathetic nervous system. It is also known that the incidence of vagal schwannoma is 2 to 3 times higher than that of sympathetic schawannoma.7 Schwannoma can compress the maternal nerve fibers which go over the tumor capsule as its size is gradually increased. Therefore, nerve paralysis may occur preoperatively. Vagal schwannoma is typically characterized by dysphagia and hoarseness. Sympathetic schwannoma is characterized by Horner's syndrome. In most cases, however, there are no symptoms, thus it is difficult to identify the neurological origin based on the physical examination.8,9
In making a differential diagnosis of the intracranial tumors, imaging studies play a key role. Particularly in cases in which schwannoma was suspected, CT is routinely performed, and such imaging diagnostic modalities as MRI, Dyanimc MRI, and angiography may be performed additionally. Imaging diagnostic modalities like CT and MRI offer great help in identifying the tumor and its correlations with surrounding vascular structures, muscles, and nerves.3 From an anatomical perspective, the carotid sheath contains the carotid artery, the internal jugular vein, and the vagus nerve. The carotid sympathetic ganglion descends medioposteiror aspect to the carotid sheath. Accordingly, when a vagal schwannoma is enlarged, the internal jugular vein is displaced laterally, and the carotid artery is displaced medially, displaying each other. In contrast, when a sympathetic schwannoma is enlarged, the carotid sheath is displaced anteriolaterally, not displaying the internal jugular vein and the carotid artery.10-12
In 1996, Furukawa, et al. performed imaging studies on nine schwannoma patients, and suggested their neurological origin prior to surgery. These authors reported an accurate diagnostic rate of 100%.3 In 2007, Saito, et al. also made an accurate diagnosis at a rate of 83% prior to surgery in 12 schwannoma patients.12 In this study, with the criteria proposed by Furukawa, et al. imaging studies were performed on all seven cases, and an accurate diagnosis was made in all cases when confirmed with later surgeries.3 Preoperative diagnosis based on imaging studies offered better understanding of the anatomical correlation between the nerve and vascular structures intraoperatively. We were also able to explain to the patients each complication of vagal schwannoma and sympathetic schwannoma which may follow the surgery preoperatively. As the authors had just experienced only one case of the schwannoma of sympathetic origin, the follow-up studies are needed for the accuracy of this study.
Schwannoma is a benign tumor that originates from the neural sheath of Schwann cells. Previously, to prevent the recurrence of tumors, radical dissection including the neuroprogenitor cells was performed. Even in cases in which recovery was achieved following the nerve transplantation or primary anastomosis, preservation of the neurological function was not to be expected. Most of the neuroprogenitor fibers do not run through schwannoma and they pass over the tumor capsule.13,14
Most schwannomas are encapsulated. In cases where the nerve fibers surround the surface of tumors, the intracapsular enucleation can be performed while preserving the nerve fibers. According to the study by Valentino, et al., intracapsular enucleation while preserving the nerve fibers preserved its function by more than 30% when compared to tumor resection with primary anastomsosis.15 The neurological functions can also be monitored using a nerve stimulator or under a microscope in performing the intracapsular enucleation.15-18
Previous studies reported the preservation rate of the neurological functions following the intracapsular enucleation to be 30-80%. In our series, the neurological function was preserved in 6 out of 7 cases. Only a minimum longitudinal incision in the capsule was made during the operation and the capsule was not connected to the nerve fibers. When the tumor was removed after making an incision, additional damages around the capsule did not occur. Moreover, when the tumor was not isolated from the capsule, we used our fingers to remove the tumor from the capsule. These cautious intracapsular enucleations could have led to the maintaining of 86% of their nerve function after the operation. In the case of patient #2, intracapsular enucleation was performed routinely; however, multiple schwannomas directly connected to the nerve fiber were observed intraoperatively. This suggests the possibility of perineurium or endoneurium origin schwannoma, and it may have caused the vocal cord paralysis examined postoperatively.
Many controversies exist regarding the recurrence rate between the total tumor resection including nerve fibers and the intracapsular enucleation. According to Zbären, et al., there was no significant difference in the recurrence rate between the total tumor resection including nerve fibers and the intracapsular enucleation. In cases where partial removal of the tumor was performed, however, the recurrence rate has been reported to rise.19 In this study, the mean follow-up period after the surgery was 3.42 years, and no recurrence has yet been noted. However, further long-term regular follow-up imaging studies are needed in this series.
In conclusion, in cases of schwannoma arising in the head and neck region, surgical resection may cause fatal nerve damage unlike other tumors. Therefore, treatments assuring the preservation of neurological functions are needed. In the current study, the neurological origins of schwannomas were predicted through preoperative imaging studies, before the surgical procedure, and we were able to explain the possible nerve damages to patients. Intracapsular enucleation was performed in all cases, and the postoperative neurological functions were preserved in most cases without recurrence. Thus, we report our treatment outcomes with a review of literature that preoperative imaging studies were effective for making an accurate diagnosis, and intracapsular enucleation was effective for preserving the neurological functions.
Figures and Tables
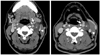 | Fig. 1Neck CT image of a vagal schwannoma patient. Tumor (asterisk) is separating the common carotid artery (white arrow) anteriorly and internal jugular vein (arrow head) posteriorly. |
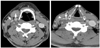 | Fig. 2Neck CT image of a sympathetic schwannoma patient. Tumor (asterisk) is anteriorly displacing the common carotid artery (white arrow) and internal jugular vein (arrow head) together without separating them. |
 | Fig. 3Operative findings (A and B) and specimen (C) of a vagal schwannoma patient. (A) A vagal schwannoma that surrounded the capsule exposed. (B) After confirming that the nerve fibers surrounded the tumor, intracapsular enucleation was performed as the tumor was carefully dissected from the capsule without any damages given to the nerve fibers. (C) A photograph of the schwannoma specimen. About 1cm sized rounded mass without the capsule is observed. |
 | Fig. 4Operative findings (A-C) and specimen (D) of a sympathetic schwannoma patient. (A) A sympathetic schwannoma that surrounded the capsule exposed. (B) A vertical incision parallel to the direction of the nerve was made on the capsule. (C) Tumor was enucleated by preserving the neural pathway using the microsurgical technique. (D) A photograph of multiple schwannoma specimens. About 6 cm sized yellowish mass like fat tissue without the capsule is observed. |
References
1. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986. 57:2006–2021.

2. Colreavy MP, Lacy PD, Hughes J, Bouchier-Hayes D, Brennan P, O'Dwyer AJ, et al. Head and neck schwannomas: a 10 year review. J Laryngol Otol. 2000. 114:119–124.
3. Furukawa M, Furukawa MK, Katoh K, Tsukuda M. Differentiation between schwannoma of the vagus nerve and schwannoma of the cervical sympathetic chain by imaging diagnosis. Laryngoscope. 1996. 106:1548–1552.

4. Fujino K, Shinohara K, Aoki M, Hashimoto K, Omori K. Intracapsular enucleation of vagus nerve-originated tumors for preservation of neural function. Otolaryngol Head Neck Surg. 2000. 123:334–336.

5. Fornaro R, Frascio M, Stabilini C. Excision of a schwannoma of the head and neck: surgical technique. G Chir. 2006. 27:428–432.
6. Moukarbel RV, Sabri AN. Current management of head and neck schwannomas. Curr Opin Otolaryngol Head Neck Surg. 2005. 13:117–122.

7. Wax MK, Shiley SG, Robinson JL, Weissman JL. Cervical sympathetic chain schwannoma. Laryngoscope. 2004. 114:2210–2213.

8. Gilmer-Hill HS, Kline DG. Neurogenic tumors of the cervical vagus nerve: report of four cases and review of the literature. Neurosurgery. 2000. 46:1498–1503.

9. de Araujo CE, Ramos DM, Moyses RA, Durazzo MD, Cernea CR, Ferraz AR. Neck nerve trunks schwannomas: clinical features and postoperative neurologic outcome. Laryngoscope. 2008. 118:1579–1582.

10. Wang CP, Hsiao JK, Ko JY. Splaying of the carotid bifurcation caused by a cervical sympathetic chain schwannoma. Ann Otol Rhinol Laryngol. 2004. 113:696–699.
11. Miller FR, Wanamaker JR, Lavertu P, Wood BG. Magnetic resonance imaging and the management of parapharyngeal space tumors. Head Neck. 1996. 18:67–77.

12. Saito DM, Glastonbury CM, El-Sayed IH, Eisele DW. Parapharyngeal space schwannomas: preoperative imaging determination of the nerve of origin. Arch Otolaryngol Head Neck Surg. 2007. 133:662–667.
13. Chang SC, Schi YM. Neurilemmoma of the vagus nerve. A case report and brief literature review. Laryngoscope. 1984. 94:946–949.

14. Saydam L, Kizilay A, Kalcioglu T, Gurer I. Ancient cervical vagal neurilemmoma: a case report. Am J Otolaryngol. 2000. 21:61–64.

15. Valentino J, Boggess MA, Ellis JL, Hester TO, Jones RO. Expected neurologic outcomes for surgical treatment of cervical neurilemomas. Laryngoscope. 1998. 108:1009–1013.

16. Charles D, Yingling CD. Cummings CW, Fredrickson JM, Harker LA, Krause CJ, Schuller DE, editors. Intraoperative monitoring of cranial nerves in neurotologic surgery. Otolaryngol-Head and Neck Surgery. 1996. St. Louis: Mosby year Book;3331–3355.
17. Pesavento G, Ferlito A, Recher G. Benign solitary schwannoma of the cervical vagus nerve. A case report with a review of the literature. J Laryngol Otol. 1979. 93:307–316.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download