Abstract
Purpose
Premixed insulin is effective to improve glycemic control; however, clinicians may be less likely to know which premixed insulin is appropriate for which patients. This study aimed to evaluate the effects of twice-daily injections of premixed insulin lispro on glycemic control in type 2 diabetic patients.
Materials and Methods
Forty type 2 diabetic patients, who had been treated with twice-daily injections of human protamine mixture 30/70 insulin for at least 12 months, were divided into two groups; one group whose blood glucose 2 hours after breakfast was greater than 200 mg/dL, was switched to lispro mix50, and the other group whose blood glucose 2 hours after breakfast < 200 was switched to lispro mix25.
Results
Glycated haemoglobin (HbA1c) significantly improved in the Mix50 group from 8.3% to 7.5% (at 12 weeks; p < 0.05), and to 7.5% (at 24 weeks; p < 0.05). On the other hand, HbA1c levels in the Mix25 group were slightly decreased from 8.1% to 7.7% at 12 weeks (p < 0.05), and to 7.9% at 24 weeks (not significant). Both postprandial plasma glucose and fasting plasma glucose levels were significantly improved in the Mix50 group, but not in the Mix25 group. Overall, 95% of subjects preferred premixed lispro insulin from human insulin in the viewpoint of the timing of insulin injection by questionnaire analysis.
Intensive insulin therapy is the best treatment for improving glycemic control to prevent the progression of diabetic microvascular complication;1 however, most type 2 diabetes patients prefer fewer daily injections to the separate use of bolus and basal components. Therefore, twice-daily injections of a mix type of insulin or a single-daily injection of long-acting insulin is widely used for diabetes. In addition, fewer daily insulin treatments can achieve good glycemic control similar to that achieved with intensive insulin therapy in patients with type 2 diabetes.2 UK Prospective Diabetes Study (UKPDS) proved that intensive treatment prevented diabetic microangiopathy complications independent of insulin usage.3 Therefore, it is important to achieve good glycemic control regardless of multiple or single insulin usage.
Humalog Mix25 (Mix25), a manufactured premixed insulin analogue containing 25% lispro insulin and 75% basal component insulin lispro-protamine, and humalog Mix50 (Mix50) containing 50% lispro and 50% NPL are widely used as a twice-daily insulin regimen.4 Compared to the human insulin mixture, twice-daily injection of Mix25 or Mix50 provided improved glycemic control with a more convenient injection just before a meal.5-7 In addition, twice-daily Mix25 therapy was reported to achieve better glycemic control than basal insulin (glargine) therapy;8 however, it is difficult to make appropriate use of Mix25 and Mix 50 for particular subjects.
In the present study, we examined the effects of two premixed analog insulins Mix25 and Mix50, by changing over from human protamine mixture 30/70 treatment. Theoretically, we switched according to the postprandial glucose level, to Mix25 for subjects with below 200 mg/dL and to Mix50 for subjects with greater than 200 mg/dL serum glucose.
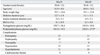
We enrolled 40 patients attending Dokkyo Medical University Hospital with type 2 diabetes and an glycated haemoglobin (HbA1c) greater than 7.5%, who had been receiving twice-daily injections of human protamine mixture 30/70 insulin therapy for at least 12 months. We excluded patients who had a stroke or cardiovascular event history. Hypoglycemic, anti-hypertensive and lipid-lowering medications were not changed during this study. The study protocol was approved by the institutional review board. All patients gave informed consent to be included in this study. The study was performed according to the guidelines in the Declaration of Helsinki.
Subjects were divided into two groups: the first group, whose postprandial plasma glucose levels (2 hours after breakfast) were greater than 200 mg/dL, was switched to lispro Mix50 (Mix50 group; n = 20); the other, whose postprandial plasma glucose levels were below 200 mg/dL, was switched to lispro Mix25 (Mix25 group, n = 20). Patient characteristics are shown in Table 1. The dosage of insulin were not changed for the first 12 weeks, but were then adjusted to achieve adequate blood glucose (< 180 mg/dL at 2 hours after breakfast in the hospital and < 130 mg/dL at fasting) for the next 12 weeks. Dietary education of diabetes was performed for 3 months before switching to premixed insulin analog. A 400 to 500 kcal meal was indicated for breakfast on the day when the patients visited the hospital. We measured fasting and 120-min postprandial levels of plasma glucose and blood HbA1c. Plasma glucose was measured by the glucose oxidase method (Medica Japan, Konosu, Japan). Blood HbA1c levels were determined by high-performance liquid chromatography (HPLC; Hi-auto A1c, HA8150; Arkray Inc., Kyoto, Japan).
After 12 weeks of treatment, to determine whether the patients feel their ideal time of insulin injections are convenient, we conducted a questionnaire survey. Responses were analyzed using a 10-point scoring system ranging from -5 (convenient timing is 30 minutes before a meal) to +5 (convenient timing is just before a meal) points.
All data were analyzed with JMP®7. The results are presented as the mean ± S.D. To compare the two groups we carried out unpaired t-test or the Mann-Whitney U-test for continuous variables, and the χ2-test or Fisher test for qualitative variables. Two-tailed p values less than 0.05 were considered significant.
In the Mix25 group, mean HbA1c levels did not change over the 12 weeks before switching; however, they were significantly decreased from 8.1% to 7.7% at 12 weeks (p = 0.003). However, there were changed to 7.9% at 24 weeks (p = 0.14), which was not significant (Table 2). Fasting plasma glucose and postprandial plasma glucose (2 after breakfast) were not significantly changed at either 12 or 24 weeks (Fig. 1).
In the Mix50 group, mean HbA1c levels did not change over the 12 weeks before switching; however, they were significantly improved from 8.3% to 7.5% at 12 weeks (p = 0.006), and to 7.5% at 24 weeks (p = 0.006) (Table 2). Fasting plasma glucose was significantly decreased from 143.0 ± 34.0 to 133.3 ± 30.5 mg/dL (p = 0.03) at 12 weeks, but remained unchanged at 134.8 ± 27.9 mg/dL at 24 weeks (p = 0.13) (Fig. 1). Postprandial plasma glucose was significantly improved from 228.8 ± 27.9 to 189.4 ± 44.3 mg/dL at 12 weeks (p < 0.001) and to 182.5 ± 41.1 mg/dL at 24 weeks (p < 0.001) (Fig. 1).
No significant changes were seen in body weight in the Mix25 group from 59.1 ± 10.6 to 59.3 ± 10.4 kg at 24 weeks, whereas the body weight of the Mix50 group increased from 59.1 ± 12.8 to 60.0 ± 13.5 kg (p = 0.003) (Table 2). There were no reported adverse reactions, such as severe hypoglycemia, during the study.
After 12 weeks of treatment, we asked the patients whether their ideal time of insulin injection is just before a meal or 30 minutes before a meal by a questionnaire survey. They were analyzed using a 10-point scoring system ranging from - 5 to + 5 points. The mean scoring was 3.5 ± 2.3; in detail, 38 patients answered that an injection just before a meal was convenient, and only 2 patients preferred an injection 30 minutes before a meal (- 4 and - 3 point) because they were used to preparing for meals and self-monitoring glucose at this time.
Our findings indicated that Mix50 treatment showed better glucose control than human 30/70 insulin therapy, especially in patients with high postprandial glucose levels. In addition, changing from premixed human insulin 30/70 to Mix50 provided better postprandial a fasting plasma glucose, resulting in improved HbA1c levels. The possibility of decreasing fasting plasma glucose by changing to Mix50 treatment was that glucose toxicity may be reduced by decreasing postprandial glucose; therefore, endogenous insulin secretion might recover,9 although the plasma c-peptide was not assessed in this study. Yamada, et al.10 proved that twice-daily injections of Mix50 could achieve better glucose control rather than premixed human insulin (30/70 and 50/50) in type 2 diabetes patients. Moreover, our study subjects were Japanese, whose main meal has a high glycemic index, such as rice. This is one reason why Mix50 treatment showed better glycemic control than human 30/70 treatment. Roach, et al.11 demonstrated that the greater proportion of rapid-acting insulin analog was more effective for carbohydrate-rich meals. It was reported that a regimen of humalog Mix50 administered three times daily before each meal maintains better glucose control than premixed human insulin 30/70 administered before breakfast and dinner.12 From our findings, we think that a regimen of humalog Mix50 administered twice-daily should be considered before stepping up to three-daily injections.
The predominant effects of Mix25 on glucose control have been reported compared to premixed human insulin 30/70.4 In particular, Mix25 decreased the postprandial glucose level better than premixed human insulin 30/70.6,13 The present study showed that overall blood glucose control was significantly improved 12 weeks after the changeover, but not at 24 weeks. Moreover, no change of fasting plasma glucose and postprandial plasma glucose (2 hours after breakfast) was observed in this study. One possibility is that faster blood glucose after breakfast could be inhibited by changing from human 30/70 insulin to Mix25. The other possibility is that postprandial blood glucose levels in the Mix25 group was not so high (< 200 mg/dL); therefore, the effect of Mix25 on postprandial glucose could not been observed clearly.
Previous studies have shown that insulin treatment for type 2 diabetes patients induced a body weight gain of 3% to 9%, depending on the study duration, diabetic control and insulin type.14 Our findings indicated that only switching from premixed human insulin 30/70 to premixed Mix50 increased by the mean baseline weight of approximately 1.6% for 24 weeks of treatment, whereas the Mix25 groups did not show any weight gain.
There were several study limitations in the present examination. One of the limitations of our present study was that the diurnal plasma glucose measurement was not performed in the outpatient setting, and therefore, the effect of changing insulin treatment on diurnal plasma glucose change was not fully assessed. In addition, two points of glucose measurement on the day when the patients visited the hospital may not provide enough information as a glucose control. Also, glucose values may be modified with food consumption the day before. Further studies using self monitoring of blood glucose or the same meal tolerance test are needed to elucidate the effects of premixed insulin on postprandial levels of plasma glucose. Second, our study design was only a changeover from human insulin 30/70 to premixed Mix25 or Mix50 selected based on postprandial plasma glucose levels (2 hours after breakfast). Although this selection was simple, the difference of diabetic severity and endogenous insulin secretion between the two groups was not considered in the selection. Therefore, if a subsequent crossover study had been done, the effect of premixed Mix25 and Mix50 insulin could be compared directly in the same patients.
In conclusion, twice-daily injections of Mix50 compared to human insulin 30/70 resulted in improved fasting and postprandial blood glucose and overall HbA1c. In addition, mixed lispro insulin therapy offered a more convenient injection time just before meals than human insulin therapy.
Figures and Tables
 | Fig. 1Effects of Mix25 and Mix50 treatment on fasting and postprandial plasma glucose. Values are the mean ± SD. *p < 0.05 vs. baseline. PG, plasma glucose; PPG, postprandial plasma glucose; FPG, fasting plasma glucose. |
References
1. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995. 28:103–117.

2. Masuda H, Sakamoto M, Irie J, Kitaoka A, Shiono K, Inoue G, et al. Comparison of twice-daily injections of biphasic insulin lispro and basal-bolus therapy: glycemic control and quality-of-life of insulin-naïve type 2 diabetic patients. Diabetes Obes Metab. 2008. 10:1261–1265.
3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.
4. Roach P, Trautmann M, Arora V, Sun B, Anderson JH Jr. Mix50 Study Group. Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lisproprotamine formulations, insulin lispro mix25 and insulin lispro mix50. Clin Ther. 1999. 21:523–534.

5. Schwartz S, Zagar AJ, Althouse SK, Pinaire JA, Holcombe JH. A single-center, randomized, double-blind, three-way crossover study examining postchallenge glucose responses to human insulin 70/30 and insulin lispro fixed mixtures 75/25 and 50/50 in patients with type 2 diabetes mellitus. Clin Ther. 2006. 28:1649–1657.

6. Mattoo V, Milicevic Z, Malone JK, Schwarzenhofer M, Ekangaki A, Levitte LK, et al. A comparison of insulin lispro Mix25 and human insulin 30/70 in the treatment of type 2 diabetes during Ramadan. Diabetes Res Clin Pract. 2003. 59:137–143.

7. Coscelli C, Iacobellis G, Calderini C, Carleo R, Gobbo M, Di Mario U, et al. Importance of premeal injection time in insulin therapy: Humalog Mix25 is convenient for improved postprandial glycemic control in type 2 diabetic patients with Italian dietary habits. Acta Diabetol. 2003. 40:187–192.

8. Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycemic control in patients with Type 2 diabetes. Diabet Med. 2005. 22:374–381.

9. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000. 23:Suppl 2. B21–B29.
10. Yamada S, Watanabe M, Kitaoka A, Shiono K, Atsuda K, Tsukamoto Y, et al. Switching from premixed human insulin to premixed insulin lispro: a prospective study comparing the effects on glucose control and quality of life. Intern Med. 2007. 46:1513–1517.

11. Roach P, Arora V, Campaigne BN, Mattoo V, Rangwala S. India Mix25/Mix50 Study Group. Humalog Mix50 before carbohydrate-rich meals in type 2 diabetes mellitus. Diabetes Obes Metab. 2003. 5:311–316.

12. Schernthaner G, Kopp HP, Ristic S, Muzyka B, Peter L, Mitteregger G. Metabolic control in patients with type 2 diabetes using Humalog Mix50 injected three times daily: crossover comparison with human insulin 30/70. Horm Metab Res. 2004. 36:188–193.

13. Koivisto VA, Tuominen JA, Ebeling P. Lispro Mix25 insulin as premeal therapy in type 2 diabetic patients. Diabetes Care. 1999. 22:459–462.

14. Mudalair SRD, Edelman SV. LeRoith D, Taylor SI, Olefsky JM, editors. Intensive insulin therapy for patients with type 2 diabetes mellitus. Diabetes Mellitus: a Fundamental and Clinical Text. 2000. Philadelphia: Lippincott Williams & Wilkins;20–38.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download