Abstract
Purpose
The inhibition of phosphodiesterase 5 produces an antinociception through the increase of cyclic guanosine monophosphate (cGMP), and increasing cGMP levels enhance the release of γ-aminobutyric acid (GABA). Furthermore, this phosphodiesterase 5 plays a pivotal role in the regulation of the vasodilatation associated to cGMP. In this work, we examined the contribution of GABA receptors to the effect of sildenafil, a phosphodiesterase 5 inhibitor, in a neuropathic pain rat, and assessed the hemodynamic effect of sildenafil in normal rats.
Materials and Methods
Neuropathic pain was induced by ligation of L5/6 spinal nerves in Sprague-Dawley male rats. After observing the effect of intravenous sildenafil on neuropathic pain, GABAA receptor antagonist (bicuculline) and GABAB receptor antagonist (saclofen) were administered prior to delivery of sildenafil to determine the role of GABA receptors in the activity of sildenafil. For hemodynamic measurements, catheters were inserted into the tail artery. Mean arterial pressure (MAP) and heart rate (HR) were measured over 60 min following administration of sildenafil.
Results
Intravenous sildenafil dose-dependently increased the withdrawal threshold to the von Frey filament application in the ligated paw. Intravenous bicuculline and saclofen reversed the antinociception of sildenafil. Intravenous sildenafil increased the magnitude of MAP reduction at the maximal dosage, but it did not affect HR response.
Neuropathic pain refers to pain due to injury or diseases of the peripheral or central nervous system.1-3 It is associated with severe, chronic sensory disturbances characterized by spontaneous pain, increased responsiveness to painful stimuli (hyperalgesia), and pain perceived in response to normally innocuous stimuli (allodynia).4,5 Prevalent symptoms in human patients include cold hyperalgesia, mechanical allodynia, and, less commonly, heat hyperalgesia. Chronic neuropathic pain remains a significant clinical problem and is often resistant to conventional analgesics, therefore, multimodal therapeutic options have been proposed.1-3
Guanylyl cyclase catalyzes the formation of cyclic guanosine monophosphate (cGMP) from GTP, leading to the synthesis of cGMP, whereas cGMP-specific phosphodiesterase catalyzes the hydrolysis of cGMP to GMP.2 Accordingly, intracellular cGMP concentrations are regulated by the action of guanylyl cyclase and the rate of degradation by cGMP-specific phosphodiesterase.6,7
It has been suggested that cGMP is involved in antinociception.8 In line with this observation, dibutyryl-cGMP and 8-bromo-cGMP showed antinociception in a modification of the Randall-Selitto hyperalgesia model and in a neuropathic pain model, respectively.7,9 Furthermore, sildenafil, a phosphodiesterase 5 inhibitor, caused antinociception in carrageenan-induced hyperalgesia, the writhing test and the formalin test.10-14 In a previous study, increasing cGMP levels with the phosphodiesterase inhibitor zaprinast enhanced the γ-aminobutyric acid (GABA) release.15 Recently, the antinociception of sildenafil was shown to be reversed by a GABAB receptor antagonist in the formalin test.16 These observations suggest that GABA receptors may be involved in the activity of sildenafil.
On the other hand, the activity of phosphodiesterase 5 was detected in smooth muscle cells of vessels.17 Also, cGMP plays important role in the regulation of vascular tone,18 and it has recently been reported that phosphodiesterase 5 is the key enzyme involved in the regulation of the cGMP-associated vascular relaxation.19 Therefore, hemodynamic change due to vasodilating effect of phosphodiesterase 5 inhibitor is expected.
In this study, we examined the effect of intravenous sildenafil in a rat neuropathic pain model and attempted to clarify the role of GABA receptors in the action of sildenafil. Furthermore, we observed the change of hemodynamics following administration of intravenous sildenafil in healthy rats.
This study proposal was reviewed and approved by the Institutional Animal Care Committee, Research Institute of Medical Science, Chonnam National University. Sprague-Dawley male rats, weighing 100-200 g, were used in all experiments. Animals were acclimated to the laboratory environment for 5-7 days before being used in the study. While in the home cage environment, the animals were allowed free access to a standard rat diet and tap water. Room temperature was maintained at 20-23℃ with 12 : 12 h light/dark cycle.
Neuropathic pain was evoked by spinal nerve ligation of experimental rats as previously described.20 Briefly, the left L5 and L6 spinal nerves of rats were isolated adjacent to the vertebral column during isoflurane anesthesia and tightly ligated with a 6-0 silk suture distal to the dorsal root ganglia. Care was taken to avoid injury of the L4 spinal nerve. Following surgery, development of neuropathic pain was evaluated daily by measuring the mechanical sensitivity of the injured paw. Animals were considered to be in neuropathic pain when they exhibited mechanical allodynia i.e., paw flinching behavior response to the application of a bending force of less than 4 g. All animals were allowed to recover for at least 1 week.
Mechanical allodynia was measured using calibrated von Frey filaments as previously described.21 Rats were placed into inverted individual plastic containers (20×12.5×20 cm) on top of a suspended wire mesh grid, and acclimated to the test chambers for 20 min. A series of eight von Frey filaments (0.4, 0.7, 1.2, 2.0, 3.6, 5.5, 8.5, and 15 g) were applied vertically to the plantar surface of the hindpaw for 5 s while the hair was bent. Brisk withdrawal or paw flinching was considered positive responses. In the absence of a response at a pressure of 15 g, animals were assigned to this cut-off value. Tests were performed in duplicate with an approximate 3 min test-free period between withdrawal responses, and their average was used. Positive responses included an abrupt withdrawal of the hind paw from the stimulus, or flinching behavior immediately following removal of the stimulus.
The following drugs were used in this study: sildenafil (phosphodiesterase 5 inhibitor), bicuculline (GABAA receptor antagonist, Sigma Aldrich Co., St. Louis, MO, USA) and saclofen (GABAB receptor antagonist, Sigma). Sildenafil was kindly provided by Korea Pfizer. All drugs were dissolved in normal saline. For the intravenous administration of these agents, the tail vein was used. For intravenous administration, drugs and saline were injected in volumes of 3 mL/kg.
In order to measure hemodynamic changes, a polyethylene-50 catheter was inserted into the tail artery under isoflurane (3-4%)/O2 anesthesia, and the rats were then restrained in a restraint cylinder. The catheter was flushed with 0.5 mL of heparinized saline. The arterial line was connected to a pressure transducer of monitor (Datex-Ohmeda AS/3, GE Healthcare, Helsinki, Finland) for continuous recording of blood pressure and heart rate.
Seven days after nerve ligation, sildenafil was administered intravenously and mechanical threshold for paw flinching was measured at 15, 30, 45, 60, 90, 120, 150, and 180 min after delivery of sildenafil. Measurement of the mechanical threshold for paw flinching was also carried out before surgery (preoperative control). The control study was performed using intravenous saline. The investigator was blind to the drug given to the experimental animals.
To examine its effect, saline and sildenafil (1, 3, 10, and 30 mg/kg, n = 37) were intravenously administered, and the mechanical threshold was measured with the above-mentioned method. Additionally, blood pressure and heart rate were measured in normal rats following intravenous administration of saline and sildenafil (1, 3, 10, and 30 mg/kg, n = 25).
To investigate the role of GABA receptors in the action of sildenafil, GABAergic antagonists were intravenously administered 10 min prior to the delivery of intravenous sildenafil, and changes of the effect of sildenafil were examined after pretreatment with GABAA receptor antagonist (bicuculline, 16 mg/kg, n = 7) or GABAB receptor antagonist (saclofen, 16 mg/kg, n = 8). The maximum doses of bicuculline and saclofen used were chosen based on previous experiments22 and the pilot study was performed to examine whether the maximum dosages of bicuculline (n = 5) and saclofen (n = 6) affected the paw withdrawal threshold or not.
Data are expressed as mean ± SEM. The time response data are presented as paw withdrawal threshold to mechanical stimulation or percentage change from the baseline mean arterial blood pressure (MAP) and heart rate (HR). The dose-response data are presented as percentage of maximal possible effect (%MPE) according to the formula.23
The dose-response data were analyzed using one-way analysis of variance with Scheffe post hoc analysis. Comparison of antagonism for the effect of sildenafil was analyzed by unpaired t-test. The baseline MAP and HR of the several groups were examined by one-way analysis of variance. The effect of sildenafil on MAP and HR was examined by repeated-measures analysis of variance. Values of p < 0.05 were considered statistically significant.
In control groups, a paw withdrawal threshold was 11-12 g. After nerve ligation, a paw withdrawal threshold was significantly decreased in a pathologic site. As shown in Fig. 1A and B, intravenous sildenafil resulted in a dose-dependent increase of the paw withdrawal threshold (p < 0.05, p < 0.01).
Intravenous GABAA antagonist (bicuculline, p < 0.01) and GABAB antagonist (saclofen, p < 0.05) reversed the antinociception of sildenafil (Fig. 2). Both antagonists themselves were not effective in ameliorating the control response.
The baseline MAP and heart rate HR were 107 ± 1 mmHg and 420 ± 3 beats/min, respectively. Furthermore, the baseline MAP and HR in the several treatment groups did not differ.
The extent of change of MAP was not statistically significant compared to baseline value over the 60 min-period following the administration of intravenous sildenafil (1, 3, 10 mg/kg). However, 30 mg/kg of sildenafil significantly augmented the extent of decrease of MAP at 5, 10 and 20 min (p < 0.05) (Fig. 3A). The extent of change of HR was not statistically significant compared with baseline value following intravenous sildenafil administration (Fig. 3B).
Neuropathic pain occurs as a result of various conditions that cause functional abnormalities or direct injury in the nervous system or many diseases such as diabetic neuropathy, postherpetic neuralgia, and trigeminal neuralgia.1-3 Central sensitization is the main contributor to the development of neuropathic pain. The related symptoms are the aberrant responses encountered in animal models and by patients (i.e., hyperalgesia and allodynia).4,5 It is estimated to afflict millions of people worldwide with neuropathic pain. A previous study reported that a population prevalence of neuropathic pain was about 8%.24 The management of neuropathic patients is complex, and responses of patients to treatments are inconsistently noted.25,26 Even with well-established neuropathic medications, effectiveness is inadequate and undesirable side effects are also troublesome.25,26 Thus, those phenomena have led to the development of treatment modality that can be used in clinical practice.
In the present study, intravenous sildenafil was found to increase the paw withdrawal threshold after spinal nerve ligation. Therefore, it is conceivable that the increased cGMP level by inhibition of phosphodiesterase 5 may contribute to the attenuation of the neuropathic pain at a systemic level.
Phosphodiesterase enzymes occur widely in biological systems and are present in mammalian tissues.27 Phosphodiesterase is an enzyme involved in the hydrolysis of cGMP, and 11 subtypes of phosphodiesterase isoenzymes have been identified on the basis of their functional characteristics, such as substrate specificity, cellular distribution and susceptibility to selective inhibitors.28 Among these isoenzymes, type 5 phosphodiesterase exerts the most significant effect on the hydrolysis of cGMP.7 Of particular interest, cGMP may play a pivotal role in the antinociceptive mechanism. Several studies have indicated that dibutyryl-cGMP, 8-bromo-cGMP and sildenafil produce antinociception in various types of noxious stimulation.7,10-14 On the other hand, intravenous GABAA antagonist (bicuculline) and GABAB antagonist (saclofen) blocked the antinociceptive effect of intravenous sildenafil in the present study. These findings suggest that both GABAA and GABAB receptors may contribute to the action of sildenafil at a systemic level.
In the hippocampus, GABA is the main inhibitory neurotransmitter, modulated by inhibitory GABA-releasing interneurons.29,30 GABA is thought to be released from the interneurons upon feed-forward inhibition by granule cells or feedback inhibition by pyramidal cells.31 On the other hand, cGMP appears to be involved in GABA release, having effects of its own as well as a part of the nitric oxide-cGMP pathway. It would therefore, appear that cGMP analog increases the synaptic GABA release to labeled paraventricular nucleus neurons.32,33 And increasing cGMP level by superfusing slices with another phosphodiesterase inhibitor (zaprinast) increases GABA release in the brain stem, while a guanylyl cyclase inhibitor (ODQ) reduces GABA release.15 Recently, it has been demonstrated that potassium channels are downstream effectors of cGMP on GABA release.33 Furthermore, the antinociception provoked by sildenafil and dibutyryl-cGMP was reversed by potassium channel blockers.10,34 These findings together suggest that intravenous sildenafil increases the cGMP level by inhibition of phosphodiesterase 5 and then induces the release of GABA through a downstream mechanism, involving potassium channels which act on GABA receptors, in turn leading to an antinociceptive effect.
As mentioned earlier, the activity of phosphodiesterase 5 was demonstrated in arterial and venous smooth muscle cells.17 Moreover, phosphodiesterase 5 activity seemed to be relevant to cGMP-linked vasodilatation.19 It is, therefore, possible that phosphodiesterase 5 inhibitor may have significant effect on hemodynamics. In this study, intravenous sildenafil at the highest dosage used decreased MAP. On the other hand, no significant change of HR response was noted after intravenous sildenafil administration. Previous studies showed modest effect of sildenafil on hemodynamics. Oral sildenafil moderately reduced blood pressure in normal humans.35 Furthermore, a significant decrease of blood pressure was transiently observed with intravenous sildenafil in healthy men, whereas HR was not affected.36 Particularly, the combination of sildenafil with nitric oxide donors can potentiate the hypotensive effect by accumulation of cGMP.37 Therefore, the concomitant use of sildenafil with any drug which serves as a nitric oxide donor is absolutely contraindicated in clinics.38
Taken together, the current study provided an important information about the signaling mechanisms through which cGMP modulates the GABA receptors. These experiments indicate that intravenous sildenafil attenuated mechanical allodynia evoked by the ligation of spinal nerve. Such antinociception may be mediated through GABAA and GABAB receptors. This new information is expected to deepen our understanding of the role of GABA receptors for the action of intravenous sildenafil and indicates a usefulness of sildenafil for neuropathic pain as a treatment strategy. Furthermore, hemodynamic effect of intravenous sildenafil should carefully be evaluated when given with other antihypertensive drugs.
Figures and Tables
Fig. 1
Temporal effect and dose response curves of intravenous saline (control) and sildenafil (n = 37) on mechanical threshold in rats following ligation of L5/6 spinal nerves. Data are presented as paw withdrawal threshold (A) and %MPE (B). Intravenous sildenafil increased the withdrawal threshold in a dose-dependent manner. Each line or bar represents mean ± SEM. BL, baseline. Compared with control, *p< 0.05, †p< 0.01.

Fig. 2
The effects of intravenous bicuculline (16 mg/kg, n = 7) and saclofen (16 mg/kg, n = 8) on the antinociception by intravenous sildenafil (30 mg/kg) in spinal nerve ligation-induced neuropathic pain. Bicuculline and saclofen were administered 10 min before the delivery of sildenafil. Data are presented as %MPE. Both bicuculline and saclofen attenuated the antinociception of sildenafil. Neither bicuculline (n = 5) nor saclofen (n = 6) alone affected the control response. Each bar represents mean ± SEM. Compared with sildenafil, *p< 0.05, †p< 0.01.

Fig. 3
Temporal effect of intravenous saline and sildenafil (n = 25) on mean arterial pressure (MAP, A) and heart rate (HR, B). Saline (control) and sildenafil were administered at time 0. Percent change of MAP and HR from baseline is plotted against time. Each line represents mean ± SEM. Compared with baseline, *p< 0.05.

ACKNOWLEDGEMENTS
This work was supported Pfizer Pharmaceuticals Korea Ltd. (Grant number: IG-KOR-020-2005).
References
1. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007. 132:237–251.

2. Finnerup NB, Sindrup SH, Jensen TS. Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharmacol. 2007. 21:129–136.

3. Wallace JM. Update on pharmacotherapy guidelines for treatment of neuropathic pain. Curr Pain Headache Rep. 2007. 11:208–214.

4. Chizh BA, Göhring M, Tröster A, Quartey GK, Schmelz M, Koppert W. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth. 2007. 98:246–254.

5. Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005. 132:193–201.

6. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.

7. Pyne NJ, Arshavsky V, Lochhead A. cGMP signal termination. Biochem Soc Trans. 1996. 24:1019–1022.

8. Ferreira SH, Nakamura M. I-Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979. 18:179–190.

9. Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001. 897:9–19.
10. Araiza-Saldaña CI, Reyes-García G, Bermúdez-Ocaña DY, Pérez-Severiano F, Granados-Soto V. Effect of diabetes on the mechanisms of intrathecal antinociception of sildenafil in rats. Eur J Pharmacol. 2005. 527:60–70.

11. Asomoza-Espinosa R, Alonso-López R, Mixcoatl-Zecuatl T, Aguirre-Bañuelos P, Torres-López JE, Granados-Soto V. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol. 2001. 418:195–200.

12. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001. 909:170–178.

13. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003. 67:150–156.

14. Mixcoatl-Zecuatl T, Aguirre-Bañuelos P, Granados-Soto V. Sildenafil produces antinociception and increases morphine antinociception in the formalin test. Eur J Pharmacol. 2000. 400:81–87.

15. Saransaari P, Oja SS. Characteristics of GABA release induced by free radicals in mouse hippocampal slices. Neurochem Res. 2008. 33:384–393.
16. Kim WM, Yoon MH, Lee HG, Han YG, Kim YO, Huang LJ, et al. GABAB receptor modulation on the antinociception of intrathecal sildenafil in the rat formalin test. Korean J Pain. 2007. 20:106–110.

17. Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999. 83:3C–12C.
18. Matsumoto T, Kobayashi T, Kamata K. Phosphodiesterases in the vascular system. J Smooth Muscle Res. 2003. 39:67–86.

19. Santos-Silva AJ, Cairrão E, Morgado M, Alvarez E, Verde I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur J Pharmacol. 2008. 582:102–109.

20. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.

21. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.

22. Blandizzi C, Bernardini MC, Natale G, Martinotti E, Del Tacca M. Peripheral 2-hydroxy-saclofen-sensitive GABA-B receptors mediate both vagal-dependent and vagal-independent acid secretory responses in rats. J Auton Pharmacol. 1992. 12:149–156.

23. Dogrul A, Ossipov MH, Lai J, Malan TP Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000. 292:115–118.
24. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007. 132:237–251.
26. Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006. 7:281–289.

27. Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990. 11:150–155.

28. Uckert S, Hedlund P, Andersson KE, Truss MC, Jonas U, Stief CG. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future. Eur Urol. 2006. 50:1194–1207.

29. Freund TF, Buzsáki G. Alterations in excitatory and GABAergic inhibitory connections in hippocampal transplants. Neuroscience. 1988. 27:373–385.

30. Frotscher M, Léránth C, Lübbers K, Oertel WH. Commissural afferents innervate glutamate decarboxylase immunoreactive non-pyramidal neurons in the guinea pig hippocampus. Neurosci Lett. 1984. 46:137–143.

31. Grudt TJ, Jahr CE. Quisqualate activates N-methyl-D-aspartate receptor channels in hippocampal neurons maintained in culture. Mol Pharmacol. 1990. 37:477–481.
32. Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004. 554:100–110.
33. Yang Q, Chen SR, Li DP, Pan HL. Kv1.1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience. 2007. 149:315–327.

34. Soares AC, Duarte ID. Dibutyryl-cyclic GMP induces perip-heral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw. Br J Pharmacol. 2001. 134:127–131.

35. Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999. 83:35C–44C.
36. Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999. 83:13C–20C.

37. Ishikura F, Beppu S, Hamada T, Khandheria BK, Seward JB, Nehra A. Effects of sildenafil citrate (Viagra) combined with nitrate on the heart. Circulation. 2000. 102:2516–2521.

38. Ravipati G, McClung JA, Aronow WS, Peterson SJ, Frishman WH. Type 5 phosphodiesterase inhibitors in the treatment of erectile dysfunction and cardiovascular disease. Cardiol Rev. 2007. 15:76–86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


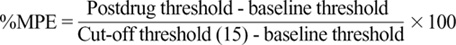
 XML Download
XML Download