Abstract
Mast cells have been regarded for a long time as effector cells in IgE mediated type I reactions and in host defence against parasites. However, they are resident in all environmental exposed tissues and express a wide variety of receptors, suggesting that these cells can also function as sentinels in innate immune responses. Indeed, studies have demonstrated an important role of mast cells during the induction of life-saving antibacterial responses. Furthermore, recent findings have shown that mast cells promote and modulate the development of adaptive immune responses, making them an important hinge of innate and acquired immunity. In addition, mast cells and several mast cell-produced mediators have been shown to be important during the development of allergic airway diseases. In the present review, we will summarize findings on the role of mast cells during the development of adaptive immune responses and highlight their function, especially during the development of allergic asthma.
Mast cells originate from pluripotent hematopoietic stem cells, which circulate as CD34+ precursors until they migrate into tissues where they mature to long living effector cells.1,2 They are present in all environmentally exposed tissues, e.g., skin, intestine and the lung, where they can be detected in epithelium and tissue, often in close proximity to blood and lymph vessels, nerves and hair follicles. In regard of biochemical, structural and functional features, different types of mast cells have been described. In humans, mast cell subtypes are named according to their protease content. MCT mast cells store tryptase in their granula in contrast to MCCT cells which express both chymase and tryptase.2,3 MCCT are found in the skin, lymph nodes and submucosa of stomach and intestine. In contrast, MCT appear predominantly in the lung and the intestinal mucosa in close proximity to other immune cells such as T cells.4,5 Two types of mast cells can be distinguished in rodents: Mast cells which reside in mucosal tissue have been named mucosal mast cells (MMC), whereas serosal mast cells can be found in connective tissue (CTMC).6 These subtypes vary in their sensitivity of activation and mediator profile.2,7
Mast cells interact with their environment by a host of mediators: Some of them are stored in mast cell granula and some are produced de novo following activation. Mast cell mediators can be divided into the following classes; a) preformed substances, b) lipid mediators and c) cytokines and chemokines. Heparin, the neutral proteases chymase, tryptase and carboxipeptidase A as well as amines like histamine are stored in preformed form in the granula and can be released within minutes following mast cell activation. Lipid mediators are generated from arachidonic acid by cyclooxygenases (COX) and prostaglandin endoperoxide synthase-1 and -2 to prostaglandin 2 (PGD2)8,9 or by 5-lipoxygenase to leukotriene A4 (LTA4). LTA4 can be further metabolized to LTB4, LTC4 or LTD4.10 Finally, mast cells are a source of a huge spectrum of cytokines and chemokines. Some cytokines can be stored in granules in preformed fashion. For example, tumor necrosis factor (TNF) can be immediately released from granules upon activation of mast cells and is also newly sensitized.11,12
Depending on the type and strength of stimulation, mast cells are able to release different mediator patterns within minutes. Mast cell activation and degranulation following IgE-mediated crosslinking of the membrane bound IgE high affinity receptor (FcεRI) is the best characterized pathway of mast cell activation.13,14 Crosslinking can be mediated by bi- or multivalent antigens, recognized by membrane-bound IgE molecules or unspecifically through super antigens. Following activation, granules fuse with the cell membrane and release their stored mediators within minutes. The metabolism of arachidonic acid and subsequent release of PGD2 and leukotrienes follows the degranulation, and finally, the de novo synthesis of cytokines and chemokines is induced.13-15 IgE-dependent mast cell activation without cross linking of FcεRI is still a controversially discussed mechanism of mast cell activation.16 During this process, single receptor-bound IgE molecules induce cytokine production even without crosslinking of FcεRI and regulate mast cell homeostasis.17,18 In addition to the FcεRI receptor, mast cells express Fcγ receptors. IgG mediated mast cell activation via these receptors plays an important role in murine models of autoimmune diseases such as arthritis and encephalitis.19,20 In mice, mainly IgG1 antibodies have been shown to contribute to Fcγ receptor-mediated activation and degranulation of mast cells.21 Mast cell can express a variety of Fcγ receptors, and Fcγ RIIB among these shows a negative regulatory effect on IgE mediated mast cell activation.22,23 Studies in Fcγ RIIB-deficient mice revealed increased anaphylactic reactions and higher susceptibility to allergic rhinitis,24,25 supporting the role of Fcγ RIIB as a negative regulator of mast cell activation.
In addition to immunoglobulins, mast cells can be activated further by exogenous and endogenous stimuli. Mast cells express a variety of receptors affiliated to innate but also adaptive immunity. Receptors of innate immunity are characterized by their ability to detect specific microbial patterns. Activation via these receptors leads to a fast immunological response, aiming at the quick clearance of the pathogen and induction of a supporting and appropriate adaptive immune response, if necessary. As mentioned before, mast cells reside in every tissue having contact to the environment, and they are one of the first cells which encounter pathogens. So far, the expression of Toll like receptors (TLR) -1,-2,-3,-4; -6, -7 and -9 as well as complement receptors and CD48 have been detected on the surface and within mast cells.26 Depending on the ligand, activation via TLR leads to distinct pattern of mediator release.27,28
The investigation of mast cell function in different immunological settings has greatly advanced with the availability of mast cell-deficient animals as a tool to analyze mast cell-dependent effects in vivo. Mast cell-deficient animals have an defective c-kit signalling either on the side of the receptor or on side of the ligand, stem cell factor (SCF).29,30 The SCF/c-kit signalling represents an important step in the development of mast cells. The WBB6F1-KitW/Wv (W/Wv) and the C57BL/6-KitWsh/Wsh mouse (Sash) represent the common used mouse strains to analyze mast cell specific effects. Both strains have mutations in the c-kit gene region, resulting in a defective expression of this receptor.31 The W/Wv is a combination of 2 mutations; KitW is a point mutation in an exon/intron border, leading to the loss of the receptor trans-membrane region,32 and KitWv is also a point mutation, resulting in a defective signalling of the receptor.33 Consequently, W/Wv mice are devoid of mast cells. However, W/Wv mice show many phenotypic abnormalities resulting from additional effects of the mutation such as anaemia, infertility, and lack of interstitial cells of Cajal. Moreover, in these animals spontaneous dilatations of the stomach, papillomas of the forestomach, dermatitis and gastric ulcers have been observed. Because of these features, the C57BL/6-KitWsh/Wsh mice have become a most popular choice as mast cell-deficient animals. The KitWsh mutation is a inversion in regulatory elements upstream of the c-kit element.34,35 Animals bearing this mutation are white and mast cell deficient, nevertheless, they are still fertile36 and not anaemic.37 For a closer analysis of mast cell function, it is possible to reconstitute mast cell deficient animals with in vitro generated mast cells.38-40 To investigate the effects of single mediators/receptors, the cells can be generated from transgenic or gene-deficient animals. Reconstitution can be performed by intradermal, intravenous or intraperitoneal application of the in vitro generated cells. Following a period of 4-8 weeks, most tissues are repopulated with mast cells.39,40 These models have helped to further unravel the role of mast cells and their mediators in innate as well as adaptive immune responses and different diseases, including allergic asthma. More recently, new approaches have been used to investigate mast cell models in vivo. Indeed, mast cell specific Cre recombinase-expressing mice have been generated by Cre expression under the control of mast cell protease 5 promoter.41 These approaches will allow to control mast cell specific gene-expression as well as targeted depletion of mast cells. However, mast cell protease 5 is expressed in CTMC but not in MMC, thus making this model unapplicable in allergic airway disease.
The induction of an adequate adaptive immune response is essential for a long lasting protection against pathogens. However, disregulated adaptive immune responses are the cause of many diseases; e.g., autoimmunity and allergy. In the last few decades, increasing evidence suggested that mast cells can induce and modulate adaptive immune responses and thereby contribute to the development of different diseases. Dendritic cells (DC) are the most specialised antigen-presenting cells of the body and the most important cells for inducing adaptive immune responses.42,43 Following activation, DC mature and migrate to the draining lymph nodes, where they act as antigen presenting cells by activating antigen specific T cells. Depending on the cytokine milieu, Th1, Th2, Th9, Th17 as well as regulatory T cells (Tregs) can be induced.42,44,45 Importantly, in several models mast cells and mast cell-produced mediators directly modulate activation and migration of DC. Indeed, mast cells induce the migration of Langerhans cells from the skin to the draining lymph nodes following activation by IgE and allergen,46 but also following IgE-independent activation.47,48 In models of contact hypersensitivity, mast cells are necessary for effective sensitization,49 and especially, mast cell-produced TNF is responsible for enhanced migration of DC from the skin to the draining lymph nodes.50 Also in the lung, sensitized wild type (WT) animals show an enhanced migration of antigen-laden DC to the draining lymph node following local challenge.51
The importance of mast cells for the induction of adaptive immune responses has further been corroborated by studies using IgE-independent mast cell activation. Application of a peptide in combination with a TLR7 ligand resulted in mast cell-dependent migration of antigen-presenting cells from the skin to regional lymph nodes and induction of a cytotoxic T cell response. Especially, cytokines IL1β and TNF produced by mast cells played a pivotal role in this setting.48
Mast cell-produced mediators can also activate antigen presenting cells. Indeed, the mast cell produced mediators histamine, PDE2 and PGD2 modulate DCs to induce the development of Th2 responses.52,53 In addition, mast cells also activate T cells by cell contact-dependent and -independent mechanisms. Through the secretion of mediators, mast cells are able to attract T cells; e.g., to the regional lymph nodes,54 thereby inducing lymph node hyperplasia.55 Moreover, mast cells are also able to directly activate T cells. Especially, mast cell-produced TNF leads to the activation of T cells.56,57 In addition, in vitro studies have shown that mast cells can process antigens and are able to present them via MHCI or MHCII complexes. Indeed, the expression of MHCI is confirmed for all mast cell subtypes and has been shown to lead to activation of CD8+ T cells in vitro.58,59 However, the expression of MHCII and upregulated costimulatory molecules remains controversial and seems to depend largely on the culture conditions.60-63 Interestingly, mast cells can release exosomes, bearing costimulatory molecules and antigen, which causes phenotypic and functional maturation of dendritic cells.64 However, many of the described phenomena have been observed only in vitro, and, therefore, it has to be shown that mast cells play a major role as antigen presenting cells in vivo.
Asthma is a chronic inflammatory disease of the airways which shows heterogenous clinical phenotypes. Approximately 8% of the adults and 14% of children in the western world are affected by asthma, making it to one of the most widespread worldwide chronic diseases. Asthma phenotypes are mainly based on clinical characteristics and inflammation patterns observed in the airways. In childhood, asthma is differentiated into transient infant wheezing, non-atopic wheezing and allergic wheezing,65 however, the disease may also develop during or after puberty.66 Irrespective of the phenotype, asthma is pathophysiologicaly characterized by three major hallmarks; airway inflammation, bronchial hyperreactivity and obstruction. Inflammation is featured by the influx of inflammatory cells; e.g., T cells, neutrophils and eosinophils. The inflammatory pattern of cell types depends on the asthma phenotype, duration and severity of disease, and treatment. In human airways, mast cells can be found adjacent to blood vessels in the lamina propria of airway mucosa. Interestingly, in patients with asthma mast cells also migrate into other structures like airway epithelium,67 the mucous glands68 and airway smooth muscle.69 This anatomical proximity to key structures involved in asthma and in vitro evidence for direct interaction between mast cells and airway smooth muscle cells suggest that mast cells play a significant role in the pathophysiology of this disease.70 Mast cells and smooth muscle cells interact in a crosstalk as mast cells can induce TGF-β1 expression in smooth muscle cells via release of β tryptase, resulting in differentiation of the muscle cells into a more contractile phenotype.71 Moreover, airway smooth muscle cells can enhance mast cell survival in a cell contact-dependent manner and can induce mast cell degranulation, representing a new antigen independent type of mast cell activation.72 Nevertheless, mast cells seem not to influence proliferation and survival of smooth muscle cells.73
In patients with allergic asthma, inhalation of an aeroallergen leads to crosslinking of membrane bound IgE via the allergen, inducing rapid release of mast cell mediators such as histamine, leukotrienes, proteases and prostagladins, which can be detected in increased concentration in the broncho-alveloar lavage (BAL) of allergen challenged patients.74-76 These mediators induce vasodilation, contraction of the smooth muscle and mucous secretion. Moreover, these mediators also lead to the late phase response which is characterized by infiltrating inflammatory cells, eosinophils, CD4+ T cells, neutrophils, mast cells and basophils which are associated with swelling of the bronchial wall and increased non-specific airway hyperresponsiveness (AHR). The important role of mast cells is underlined by studies on histamine and leukotriene receptor antagonists or anti-IgE antibodies, which completely ameliorate the development of the early phase and also partly the late phase response.77-79
Despite these results, the role and function of mast cells in the initial development of allergic asthma cannot be investigated in humans for ethical reasons, and therefore, animal studies are needed to assess molecular and cellular interactions responsible for the induction and exacerbation of the disease. Especially, rodent (mouse and rat) models have been used to analyze the pathomechanisms of allergic airway disease. These models can mimic many features of human asthma. However, due to profound differences in physiology between mice and men, not all the findings with murine models can uncritically be transferred to human situation. Yet, murine models helped to reveal many pathophysiologically important aspects regarding the role of mast cells in the development of allergic asthma.80,81
Using murine models, the role of mast cells in the induction of an allergic airway disease has been intensively investigated. Airway hyperresponsiveness and inflammation are comparable between wild type mice and mice lacking either mast cells, B cells, IgE or FcεRI, when animals are sensitized systemically by injection of allergen in combination with an adjuvant and subsequently challenged via the airways.82-85 Yet, some studies showed that mast cells are necessary for an enhanced influx of eosinophils into the lung,86,87 for the induction of an increased airway hyperresponsiveness88 or for the induction of subepithelial fibrosis.89 In many immunisation protocols, aluminum hydroxide (Alum) is used as an adjuvant, which acts via NALP3 inflammasome90 and induces a strong Th2 polarisation in the system.91 Furthermore, alum has direct effects on mast cells and macrophages.92 In contrast, however, studies using sensitization protocols without additional adjuvant showed that mast cells are necessary for the induction of allergic airway disease.93 The important function of FcεRI for development of AHR and inflammation was further identified.94,95 In addition, using mast cell-deficient animals and engraftment with bone marrow-derived mast cell (BMMCs), the function of mast cell-derived mediators was assessed.
Indeed, mast cell-deficient mice which were reconstituted with BMMCs from TNF-deficient donors showed less inflammation and AHR compared to reconstituted animals which received BMMCs from wild type donors.96,97 These findings have been supported by a variety of murine and also human studies. The expression of TNF is upregulated in the airways of asthmatics in comparison to healthy subjects,98 and intratracheal application of TNF in healthy subjects induces AHR and inflammation.99,100 Murine models confirmed that TNF is important for the induction of mucus gene expression101 and necessary for the late phase response.102 Thus, TNF-deficient animals fail to develop an allergic airway disease, compared to WT animals.103
Another important mast cell-produced mediator is histamine which acts on different cell types via four distinct receptors (HR).104 Depending on the expression level of the receptors and the cell type, histamine can have different effects with pro- but also anti-inflammatory patterns. In regard to DC activation, H1R and H3R induce pro-inflammatory responses with increased antigen presentation, cytokine production and Th1 priming activity, whereas activation of H2R induces IL-10 secretion and a regulatory DC phenotype.105 In T cells, depending on the receptor expression pattern histamine can induce the production of Th1 cytokines such as IFN-γ or Th2-specific cytokines like IL-4 and IL-13.106 Recently, it has also been demonstrated that pro-inflammatory effects of mast cell-derived histamine might be mediated by suppressing CD4+ CD25+ regulatory T cells.107 In allergic airway disease, Bryce, et al.108 demonstrated an important role of histamine acting via H1 receptor. Indeed, H1-receptor-deficient animals were not able to allergic airway disease following sensitization and challenge. Especially, H1 receptor-deficient animals showed a defect in T cell migration into the lung. Furthermore, H4 receptor also seems to play an important role in the histamine-dependent induction of allergic airway disease.109 Consequently, novel H4 receptor antagonists have been developed and have been shown to be effective in suppressing the development of allergic airway disease in murine models.110
Mast cells are also capable to synthesize different lipid mediators. Interestingly, animals over-expressing prostaglandin D2(PGD2) develop increased airway inflammation and Th2 cytokine production following sensitization and challenge in comparison to WT animals.111 Furthermore, inhalation of PGD2 just before airway challenge results in worsening of allergic airway disease,112 whereas blocking PGD2 synthesis decreases inflammation.113 The effects induced by PGD2 are dependent on the respective receptor. Two receptors have been described; PGD2 receptor 1 (DP1) which has pro inflammatory effects,114 while the function of PGD2 receptor 2 or chemo-attractant homologous receptor expressed on Th2 cells (DP2 or CRTH2) is more controversial. In allergic airway disease models, CRTH2 agonists increased airway inflammation whereas the receptor antagonists decreased it.115,116 In mice deficient in CRTH2, however, increased numbers of eosinophils and higher amounts of IL-5 were detected following allergen sensitization and challenge, compared to wild-type littermates.117 However, human Th2 cells which express CRTH2 show increased production of Th2 cytokines following exposure to PGD2 in the absence of costimulation.118 This suggests that blockade of the CRTH2 receptor might be an attractive approach for the treatment of allergic asthma, and indeed, CRTH2 antagonists are being tested in clinical trials.
Leukotrienes are also mast cells-produced lipid mediators, which affect the development of allergic airway disease.119,120 Arachidonic acid represents the source material for it's synthesis. A multiprotein complex which includes 5-lipoxygenase (5-LO) initiates the transformation of free arachidonic acid to reactive leukotriene A4 that can be further metabolized to different leukotriene subtypes.121 LTC4 synthase represents the key enzyme for the induction of cys leukotrienes (leukotriene C4; D4 and E4), whereas LTB4 conversion is initiated by LTA4 hydrolase.122-124 Several studies identified LTs to be important for the recruitment of T cells125-127 and dendritic cells.128 Moreover, LTC4 and LTD4, acting via the CysLT2 receptor, seem to be important for fibrosis and vascular injury.129,130 Inhalatory application of LTE4, but not LTD4, induces the influx of inflammatory cells into the lung.131 Taken together, prostaglandins as well as leukotrienes seem to play crucial roles in modulating and attracting immunocompetent cells. Thus, these mediators could be key players in mast cell-dependent modulation of adaptive immune responses.
Thymic stromal lymphopoitein (TSLP) is a cytokine produced mainly by keratinocytes, epithelial and stromal cells. TSLP expression in the lung is upregulated in patients with asthma,132 and mice deficient for the receptor do not develop allergic airway disease.133,134 TSLP was also shown to induce DC activation, leading to a Th2 inducing phenotype in human as well as in mice.133 Therefore, TSLP is an important factor in modulating adaptive immune responses towards Th2. Interestingly, mast cells express the TSLP receptor, and exposure to TSLP leads to expression of Th2 cytokines.135 In addition, mast cells can produce high levels of TSLP, following IgE-mediated activation,136 and are vital for the induction of TSLP expression following allergen exposure.137
Recent evidence suggests that mast cells not only play an important role in the induction of allergic airway disease in already sensitized hosts, but also are involved directly in the induction of specific T cell responses to aeroallergens. Following exposure to aeroallergens, the usual outcome is tolerance, because most allergens are immunologically inert proteins, and inflammation does not develop even following chronic exposure. Resident pulmonary DCs are usually in a state specialized to internalize foreign antigens, but not able to activate naïve T cells. Stimulation of DCs with additional factors like ligands for TLR eventually leads to their activation, migration to the regional lymphatic tissue and induction of a specific T cell response by antigen presentation and increased expression of co-stimulatory molecules.138 There is increasing in vitro evidence, that activation of mast cells can modulate the differentiation of DCs to a Th2 biased phenotype by histamine and prostaglandin secretion.139,140 Also, several studies in vivo suggest the involvement of mast cells in T cell priming following inhaled allergen exposure. In addition to the administration of a protein allergen, low doses of bacterial lipopolysaccharide (LPS) can induce sensitization to the allergen mediated by TLR-4 and production of TNF.141 Indeed, following intranasal challenge with allergen in conjunction with low-dose LPS, mast cell-deficient mice fail to develop sensitization to the allergen, demonstrating that IgE-independent activation of mast cells is involved in the initiation of a T cell response following inhaled allergen exposure.142
Other protein allergens, like house dust mite allergens (D. pteronyssinus, D. farinae) have the potential to directly induce rapid IgE-independent release and de novo synthesis of mast cell mediators.143,144 In vitro assays of D. farinae-stimulated mast cells show that their supernatants attract monocytes and T cells, support T cell proliferation and promote Th2 cell development. Inhaled exposure to house dust mite can induce Th2 sensitization and an influx of activated Th2 effector cells into the lung in vivo.145 In further support of the role of mast cells in this process, administration of sodium cromoglycate, a mast cell stabilizer, during repeated house dust mite allergen exposure not only suppresses the production of acute mast cell mediators but also attenuates airway inflammation following repeated D. farinae exposure.144
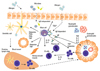
In summary, mast cells play an important role in innate and adaptive immunity. This is mainly due to their ability to produce a variety of proinflammatory and immunomodulatory mediators. Upon their activation, they promote the migration of antigen-exposed antigen-presenting cells to the regional lymph nodes. Consequently, mast cells, at least under certain conditions, are able to strongly promote the development of specific T cell responses and are also able to shape them. In the context of allergic asthma, mast cells have been shown to initiate and promote airway inflammation and AHR in murine models and human disease, which also involves secretion of several mast cell-produced mediators (Fig. 1). Taken together, these findings clearly demonstrate that mast cells are not only mere effector cells during allergic reactions, but also have a complex role in the induction and regulation of adaptive immune responses. In regard to allergic sensitization, the activation of mast cells seems to be an important regulatory step for the development of specific T cell responses to the allergen. Therefore, modulation of mast cell activation could be a potential therapeutic strategy for the prevention and treatment of allergic disease.
Figures and Tables
Fig. 1
Role of mast cells during sensitization to an aeroallergen and during challenge with allergen. Inhalation of an aeroallergen in combination with exposure to an alternative/IgE-independent mast cell-activating stimulus leads to the migration of local dendritic cells to the regional lymph nodes and there to an induction of a Th2 response. In addition, allergen exposure results in allergen IgE-dependent mast cell activation and leads to an increased chemotaxis of inflammatory cells as well as local T cell activation. TSLP, thymic stromal lymphopoitein; PGD2, prostaglandin D2; PGE2, prostaglandin E2; TNF, tumor necrosis factor.
References
1. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002. 2:773–786.

3. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986. 83:4464–4468.

4. Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987. 138:4381–4386.
5. Goldstein SM, Kaempfer CE, Proud D, Schwartz LB, Irani AM, Wintroub BU. Detection and partial characterization of a human mast cell carboxypeptidase. J Immunol. 1987. 139:2724–2729.
6. Enerbäck L, Pipkorn U, Granerus G. Intraepithelial migration of nasal mucosal mast cells in hay fever. Int Arch Allergy Appl Immunol. 1986. 80:44–51.
7. Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005. 23:749–786.

8. Murakami M, Matsumoto R, Austen KF, Arm JP. Prostaglandin endoperoxide synthase-1 and -2 couple to different transmembrane stimuli to generate prostaglandin D2 in mouse bone marrow-derived mast cells. J Biol Chem. 1994. 269:22269–22275.

9. Urade Y, Ujihara M, Horiguchi Y, Igarashi M, Nagata A, Ikai K, et al. Mast cells contain spleen-type prostaglandin D synthetase. J Biol Chem. 1990. 265:371–375.

11. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990. 346:274–276.
12. Young JD, Liu CC, Butler G, Cohn ZA, Galli SJ. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci U S A. 1987. 84:9175–9179.

14. Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999. 402:B24–B30.
15. Mayr SI, Zuberi RI, Liu FT. Role of immunoglobulin E and mast cells in murine models of asthma. Braz J Med Biol Res. 2003. 36:821–827.

16. Schweitzer-Stenner R, Pecht I. Death of a dogma or enforcing the artificial: monomeric IgE binding may initiate mast cell response by inducing its receptor aggregation. J Immunol. 2005. 174:4461–4464.

17. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002. 2:773–786.

18. Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001. 14:801–811.

19. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002. 297:1689–1692.

20. Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J Immunol. 2003. 170:1630–1634.
21. Vaz NM, Prouvost-Danon A. Behaviour of mouse mast cells during anaphylaxis in vitro. Prog Allergy. 1969. 13:111–173.
22. Daëron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995. 95:577–585.

23. Daëron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, et al. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995. 3:635–646.

24. Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996. 379:346–349.

25. Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999. 189:1573–1579.

26. Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008. 213:251–260.

27. Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994. 152:5468–5476.
28. Dvorak AM. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem Immunol Allergy. 2005. 85:135–184.

29. Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978. 52:447–452.

30. Kitamura Y, Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979. 53:492–497.

31. Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988. 55:185–192.

33. Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990. 9:1805–1813.
34. Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, et al. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993. 118:705–717.

35. Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008. 173:1693–1701.

36. Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982. 39:315–322.

37. Tono T, Tsujimura T, Koshimizu U, Kasugai T, Adachi S, Isozaki K, et al. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood. 1992. 80:1448–1453.

38. Tsai M, Tam SY, Wedemeyer J, Galli SJ. Mast cells derived from embryonic stem cells: a model system for studying the effects of genetic manipulations on mast cell development, phenotype, and function in vitro and in vivo. Int J Hematol. 2002. 75:345–349.
39. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005. 167:835–848.

40. Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit (W-sh) sash mice. Clin Exp Allergy. 2005. 35:82–88.

41. Scholten J, Hartmann K, Gerbaulet A, Krieg T, Müller W, Testa G, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008. 17:307–315.
42. Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000. 106:551–559.
43. Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001. 193:51–60.

44. van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, et al. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of Th2 effector responses in a mouse model of asthma. J Allergy Clin Immunol. 2004. 114:166–173.
45. Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008. 123:326–338.
46. Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol. 2004. 173:5275–5282.
47. Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006. 177:1755–1762.

48. Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood. 2007. 110:946–953.

49. Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004. 20:381–392.

50. Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006. 176:4102–4112.

51. Reuter S, Dehzad N, Martin H, Heinz A, Castor T, Sudowe S, et al. Mast cells induce migration of dendritic cells in a murine model of acute allergic airway disease. Int Arch Allergy Immunol. 2010. 151:214–222.

52. McIlroy A, Caron G, Blanchard S, Frémaux I, Duluc D, Delneste Y, et al. Histamine and prostaglandin E up-regulate the production of Th2-attracting chemokines (CCL17 and CCL22) and down-regulate IFN-gamma-induced CXCL10 production by immature human dendritic cells. Immunology. 2006. 117:507–516.

53. Theiner G, Gessner A, Lutz MB. The mast cell mediator PGD2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006. 211:463–472.

54. Wang HW, Tedla N, Lloyd AR, Wakefield D, McNeil PH. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Invest. 1998. 102:1617–1626.

55. McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003. 4:1199–1205.
56. Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005. 102:6467–6472.

57. Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006. 176:2238–2248.

58. Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009. 31:665–676.
59. Malaviya R, Twesten NJ, Ross EA, Abraham SN, Pfeifer JD. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996. 156:1490–1496.
60. Gauchat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993. 365:340–343.

61. Poncet P, Arock M, David B. MHC class II-dependent activation of CD4+ T cell hybridomas by human mast cells through superantigen presentation. J Leukoc Biol. 1999. 66:105–112.

62. Grabbe J, Karau L, Welker P, Ziegler A, Henz BM. Induction of MHC class II antigen expression on human HMC-1 mast cells. J Dermatol Sci. 1997. 16:67–73.

63. Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009. 182:4686–4695.

64. Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003. 170:3037–3045.

65. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003. 111:661–675.

66. De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinoni A, Giammanco G. Italian Study on Asthma in Young Adults study group. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002. 110:228–235.
67. Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994. 10:471–480.

68. Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax. 2002. 57:677–682.

69. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002. 346:1699–1705.

70. Yang W, Kaur D, Okayama Y, Ito A, Wardlaw AJ, Brightling CE, et al. Human lung mast cells adhere to human airway smooth muscle, in part, via tumor suppressor in lung cancer-1. J Immunol. 2006. 176:1238–1243.

71. Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, et al. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol. 2008. 181:5001–5007.

72. Hollins F, Kaur D, Yang W, Cruse G, Saunders R, Sutcliffe A, et al. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol. 2008. 181:2772–2780.

73. Kaur D, Hollins F, Saunders R, Woodman L, Sutcliffe A, Cruse G, et al. Airway smooth muscle proliferation and survival is not modulated by mast cells. Clin Exp Allergy. 2010. 40:279–288.

74. Murray JJ, Tonnel AB, Brash AR, Roberts LJ 2nd, Gosset P, Workman R, et al. Prostaglandin D2 is released during acute allergic bronchospasm in man. Trans Assoc Am Physicians. 1985. 98:275–280.
75. Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, et al. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991. 144:51–58.
76. Casale TB, Wood D, Richerson HB, Zehr B, Zavala D, Hunninghake GW. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Invest. 1987. 80:1507–1511.

77. Roquet A, Dahlén B, Kumlin M, Ihre E, Anstrén G, Binks S, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med. 1997. 155:1856–1863.
78. Hamilton A, Faiferman I, Stober P, Watson RM, O'Byrne PM. Pranlukast, a cysteinyl leukotriene receptor antagonist, attenuates allergen-induced early- and late-phase bronchoconstriction and airway hyperresponsiveness in asthmatic subjects. J Allergy Clin Immunol. 1998. 102:177–183.
79. Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997. 155:1828–1834.

80. Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int Arch Allergy Immunol. 2004. 135:173–186.
81. Boyce JA, Austen KF. No audible wheezing: nuggets and conundrums from mouse asthma models. J Exp Med. 2005. 201:1869–1873.
82. Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997. 186:449–454.

83. Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999. 21:480–489.

84. Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, et al. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci U S A. 1997. 94:1344–1349.

85. MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999. 20:379–387.

86. Ogawa K, Kaminuma O, Kikkawa H, Kameda R, Ikezawa K, Suko M, et al. Primary role of CD4+ T cells and supplemental role of mast cells in allergic pulmonary eosinophilia. Int Arch Allergy Immunol. 1999. 120:Suppl 1. 15–18.

87. Kung TT, Stelts D, Zurcher JA, Jones H, Umland SP, Kreutner W, et al. Mast cells modulate allergic pulmonary eosinophilia in mice. Am J Respir Cell Mol Biol. 1995. 12:404–409.

88. Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000. 164:3855–3861.
89. Masuda T, Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, et al. Mast cells play a partial role in allergen-induced subepithelial fibrosis in a murine model of allergic asthma. Clin Exp Allergy. 2003. 33:705–713.

90. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008. 453:1122–1126.

91. Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999. 163:6448–6454.
92. McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009. 183:4403–4414.

93. Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000. 192:455–462.

94. Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006. 116:1633–1641.

95. Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004. 172:6398–6406.

96. Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and Th2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007. 120:48–55.

97. Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008. 31:773–782.

98. Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994. 10:471–480.

99. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995. 152:76–80.

100. Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002. 57:774–778.

101. Busse PJ, Zhang TF, Srivastava K, Lin BP, Schofield B, Sealfon SC, et al. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005. 116:1256–1263.
102. Choi IW, Kim S, Kim YS, Ko HM, Im SY, Kim JH, et al. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol. 2005. 116:537–543.

103. Nakae S, Lunderius C, Ho LH, Schäfer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy Clin Immunol. 2007. 119:680–686.

104. Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005. 137:82–92.

105. Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001. 108:1865–1873.

106. Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001. 413:420–425.

107. Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009. 183:3014–3022.

108. Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006. 116:1624–1632.
109. Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006. 176:7062–7070.

110. Cowden JM, Riley JP, Ma JY, Thurmond RL, Dunford PJ. Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respir Res. 2010. 11:86.
111. Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2002. 168:443–449.

112. Honda K, Arima M, Cheng G, Taki S, Hirata H, Eda F, et al. Prostaglandin D2 reinforces Th2 type inflammatory responses of airways to low-dose antigen through bronchial expression of macrophage-derived chemokine. J Exp Med. 2003. 198:533–543.
113. Oguma T, Asano K, Shiomi T, Fukunaga K, Suzuki Y, Nakamura M, et al. Cyclooxygenase-2 expression during allergic inflammation in guinea-pig lungs. Am J Respir Crit Care Med. 2002. 165:382–386.
114. Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000. 287:2013–2017.
115. Spik I, Brénuchon C, Angéli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005. 174:3703–3708.
116. Uller L, Mathiesen JM, Alenmyr L, Korsgren M, Ulven T, Högberg T, et al. Antagonism of the prostaglandin D2 receptor CRTH2 attenuates asthma pathology in mouse eosinophilic airway inflammation. Respir Res. 2007. 8:16.
117. Chevalier E, Stock J, Fisher T, Dupont M, Fric M, Fargeau H, et al. Cutting edge: chemoattractant receptor-homologous molecule expressed on Th2 cells plays a restricting role on IL-5 production and eosinophil recruitment. J Immunol. 2005. 175:2056–2060.

118. Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005. 175:6531–6536.

119. Henderson WR Jr, Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996. 184:1483–1494.

120. Irvin CG, Tu YP, Sheller JR, Funk CD. 5-Lipoxygenase products are necessary for ovalbumin-induced airway responsiveness in mice. Am J Physiol. 1997. 272:L1053–L1058.

121. Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983. 220:568–575.

122. Köller M, Brom J, Raulf M, König W. Cilastatin (MK 0791) is a potent and specific inhibitor of the renal leukotriene D4-dipeptidase. Biochem Biophys Res Commun. 1985. 131:974–979.

123. Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A. 1994. 91:7663–7667.

124. Orning L, Hammarström S. Inhibition of leukotriene C and leukotriene D biosynthesis. J Biol Chem. 1980. 255:8023–8026.

125. Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, et al. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005. 174:4979–4984.

126. Taube C, Miyahara N, Ott V, Swanson B, Takeda K, Loader J, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006. 176:3157–3164.

127. Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, et al. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005. 175:4217–4225.

128. Robbiani DF, Finch RA, Jäger D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000. 103:757–768.

129. Uzonyi B, Lötzer K, Jahn S, Kramer C, Hildner M, Bretschneider E, et al. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci U S A. 2006. 103:6326–6331.

130. Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004. 279:46129–46134.

131. Gauvreau GM, Parameswaran KN, Watson RM, O'Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001. 164:1495–1500.

132. Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005. 174:8183–8190.

133. Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005. 202:829–839.

134. Li YL, Li HJ, Ji F, Zhang X, Wang R, Hao JQ, et al. Thymic stromal lymphopoietin promotes lung inflammation through activation of dendritic cells. J Asthma. 2010. 47:117–123.

135. Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007. 20:253–258.

136. Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009. 34:425–435.

137. Miyata M, Nakamura Y, Shimokawa N, Ohnuma Y, Katoh R, Matsuoka S, et al. Thymic stromal lymphopoietin is a critical mediator of IL-13-driven allergic inflammation. Eur J Immunol. 2009. 39:3078–3083.

138. Hammad H, Lambrecht BN. Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol. 2006. 118:331–336.

139. Gosset P, Pichavant M, Faveeuw C, Bureau F, Tonnel AB, Trottein F. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: impact on the T cell response. Eur J Immunol. 2005. 35:1491–1500.

140. Kitawaki T, Kadowaki N, Sugimoto N, Kambe N, Hori T, Miyachi Y, et al. IgE-activated mast cells in combination with pro-inflammatory factors induce Th2-promoting dendritic cells. Int Immunol. 2006. 18:1789–1799.

141. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002. 196:1645–1651.

142. Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, et al. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci U S A. 2006. 103:2286–2291.

143. Machado DC, Horton D, Harrop R, Peachell PT, Helm BA. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 1996. 26:2972–2980.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download