Abstract
Purpose
Community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) infections are increasing. Although gentamicin (GEN) is usually susceptible against CA-MRSA, GEN is rarely considered for treatment as monotherapy. We employed an in vitro pharmacodynamic model (IVPDM) to compare efficacies of GEN against CA-MRSA with two dosing regimens [thrice-daily (TD), once-daily (OD)].
Materials and Methods
Using two strains of CA-MRSA, we adopted IVPDM comprised of two-compartments with a surface-to-volume ratio of 5.34 cm-1. GEN regimens were simulated with human pharmacokinetic data of TD and OD. Experiments were performed over 48 hours in triplicate for each strain and dosing regimen.
Results
MICs of GEN for YSSA1 and YSSA15 were 1 and 2 mg/L, respectively. In OD, indices of peak/MIC were > 8.6 at least, in contrast to < 6.4 in TD. A ≥ 3-log10 reduction in CFU/mL was demonstrated prior to 4 hours in TD and OD, and continued until 8 hours for both strains. However, reductions in the colony counts at 24 and 48 hours were significantly larger for OD compared to TD in both strains (p < 0.001). During TD, resistance developed in YSSA1 and small colony variants (SCVs) were documented in YSSA15. No resistance or SCVs were observed during OD in both strains.
Recent reports have described an increasing incidence of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA); this group differs from the health care-associated (HA) MRSA and has become a major concern worldwide.1,2 Most cases of CA-MRSA have been associated with the absence of multi-drug resistance in contrast to HA-MRSA cases. A common strategy for treating serious MRSA infection is to use glycopeptide with or without aminoglycosides.3-5 However, gentamicin (GEN) is rarely considered for the treatment of CA-MRSA in combination or monotherapy due to questions of efficacy based on discordant in vitro studies, lack of supporting clinical data, and risk of the development of nephrotoxicity and resistance; although it is usually susceptible against CA-MRSA.2,6-9 Another recently additional concern is the development of small colony variants (SCVs)-subpopulation with reduced susceptibility-with incomplete inhibitory concentrations of GEN, which may be responsible for recurrence or persistence of infection.10-12
It is needed to ascertain whether characteristics of traditional thrice-daily (TD) or once-daily (OD) administration of GEN is working against CA-MRSA as reported in cases of gram-negative rods infection.13,14
In this study, we have tried to evaluate the efficacy and to monitor the development of resistance or SCVs during TD administration of GEN in an in vitro pharmacodynamic model (IVPDM). In addition, we compared the efficacy of TD to OD dosing of GEN with considering optimal pharmacodynamic indices.
Two prototypes of GEN susceptible CA-MRSA strains, previously reported from our laboratory, were used in this study. One (YSSA1) is the multilocus sequence type (ST) 72-staphylococcal chromosome cassette mec (SCCmec) type IVA-spa t324-group I agr without Panton-Valentine leukocidin (PVL) gene, which is considered representative of predominant CA-MRSA clonal type in Korea. The other (YSSA15) is the USA 300 like strain characterized by ST8-SCCmec type IV-spa type t008-group I agr and PVL positive.15,16
Broth microdilution MICs were performed in accordance with the Clinical and Laboratory Standards Institute Guidelines.17 GEN was purchased (Sigma Chemical, St. Louis, MO, USA). Its solution stock, with a concentration of 1 mg/mL, was frozen at -70℃. Cation-adjusted Mueller-Hinton broth (Difco, Detroit, MI, USA) was used for susceptibility testing and IVPDM. The colony counts were determined using tryptic soy agar (TSA) (Difco) plates.
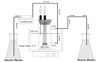
Experiments were performed in an IVPDM, as previously described (Fig. 1).18,19 Briefly, it was comprised of a central compartment consisting of a dual-port 100 mL chromatography column water jacket into which a dialyzer (Spectrum, Philadelphia, PA, USA) was suspended from a rubber stopper. The dialyzer (peripheral compartment) held approximately 6 mL of fluid. The dialyzer membrane was composed of a synthetic regenerated cellulose ester with a pore size of 0.004 µm and an effective surface area-to-volume ratio of 5.34 cm-1. Both the central and peripheral compartments of the model were fitted with needles for withdrawing samples. GEN was administered via syringes connected to the central compartment. The simulated GEN regimens were as follows: 80 mg every 8 hours (q8h, peak concentration 5 mg/L, half-life 2 hours) and 240 mg every 24 hours (q24h, peak concentration 15 mg/L, half-life 2 hours).4,20 Before starting the experiment, 10 minutes passed before reaching the equilibrium of GEN concentration between central and peripheral compartment. A total of 6 mL of bacteria (-5×106 CFU/mL) was injected into the peripheral compartment after overnight incubation and dilution of bacteria. All experiments were performed over 48 hours in triplicate for each strain. Growth controls were also tested.
Pharmacokinetic samples of GEN were taken from the central compartment at 0.25, 1, 2, 4, 6, 8, and 24 hours, after administration and measured by fluorescence polarization immunoassay (Abbott Diagnostics TDx, Irving, TX, USA); inter-day and intra-day coefficients of variation were less than 10% for all standards.
Samples for colony counts (100 µL) were taken from peripheral compartment at 0, 1, 2, 4, 8, 24, 30, and 48 hours, diluted in 0.9 mL of saline solution, and then plated on TSA. The media was cultured at 37℃ for 24 hours, and the number of colonies were measured and averaged. The lower limit of detection was 2 log10 CFU/mL. Carryover phenomena were avoided by multiple serial saline dilutions. Bactericidal activity (99.9% kill) was defined as a ≥ 3-log10 CFU/mL reduction in colony count from the starting inoculum at 24 hours. Samples (100 µL) from each time point were also plated on to TSA containing an antibiotic concentration of four and eight times of the MIC for each organism and were incubated for 48 hours at 37℃ in order to monitor the development of resistance. When resistance was suspected, post-exposure MICs were re-evaluated. Briefly, SCVs were identified by the following phenotypical characteristics: 1) slow growth, 2) small colonies, 3) decreased pigmentation, and 4) weak hemolysis on Columbia agar (Conda, Madrid, Spain). Suspected SCVs were also incubated with 5% CO2 over 48-72 hours. Small colony forming S. aureus like species testing was completed by the API ID 32 Staph system (bioMerieux Ltd, Marcy-L'Etoile, France) and nuc gene polymerase chain reaction (PCR).10,19,21
The pre-exposure MICs for YSSA1 and YSSA15 were 1 and 2 mg/L, respectively. The actual peaks were 6.4 ± 0.8 in TD and 17.2 ± 1.0 mg/L for OD dosing with measured half-lives of 2.2 ± 0.2 hour. All simulations achieved peak concentrations and measured half-lives were within 10% of targeted values. Pharmacodynamic indices were shown in Table 1. AUC0-24/MIC values were not statistically different according to the dosing regimens in each strain. Both strains had peak/MIC indices of more than 8.6 in OD regimen at least. However, less values (< 6.4) of peak/MIC were observed in TD dosing.
Colony counts at 0 hour were in the range of 6.42-6.90 log10 CFU/mL. Results of the experiments with YSSA1 and YSSA15 are presented in Fig. 2. A ≥ 3-log10 reduction in CFU/mL was demonstrated prior to 4 hours in TD and OD regimen for both strains and continued until 8 hours. The slopes of bacterial killing were not different between OD and TD regimens in both regimens during 8 hours. Reductions in the colony counts at 24 and 48 hours were significantly larger for OD compared to TD in both strains (p < 0.001). For YSSA1, resistance developed with re-growth after 24 hours during TD. The post-exposure MIC at 24 hours was 16 mg/L, and persisted for 48 hours. For OD, the bacteria were eradicated by 8 hours, and still undetectable at the end of the experiment. For YSSA15, re-growth of the colonies were shown beyond the 8 hours time point and SCVs were documented starting at 30 hours of the TD therapy with the similar number of normal colonies, but none could be documented in the OD regimen for the entire experiment. No colony was observed after eradication 4 hours during OD dosing. The SCV had a GEN MIC greater than 512 mg/L.
Aminolglycosides are known to concentration-dependent killers with post-antibiotic effect (PAE) in Gram negative organisms.13,14 The superiority of OD aminoglycoside regimens to better suppress the emergence of resistance has been demonstrated.22 It is also reported for GEN that the period of PAE and adaptive resistance by several Gram negative organisms were related to the peak concentration of the aminoglycosides.14,23,24 Thus, the aminoglycoside peak concentration dependent effect on resistance, optimized by OD dosing in contrast to more frequent dosing, has been known. However, the relationship between concentration and rate of bacterial kill or resistance development of GEN with Gram-positive organisms such as S. aureus, and their differences with Gram negative organisms are not well established.
In this experiment, although thorough dose-fractionation studies were not completely performed, the bacterial killing rates (slope of curve) out to the 8 hours time point seemed not to be different between these two regimens as previously reported.25,26 However, OD dosing over 24-48 hours had superior killing effects than TD as the same thing happened in Gram negative organisms. Considering the early rapid killing characteristics of GEN, hypotheses of these discordances (i.e., non-concentration dependent characteristics on early phase, and then concentration dependent killing) would the be rapid killing of susceptible populations during the early period and then re-growth of resistant subpopulations later. For further understanding of PD characteristics of GEN against CA-MRSA, complete dose-fractionation studies with strains of variable MICs are warranted.
Munckhof, et al.6 reported that high-level resistance developed in vitro in CA-MRSA after exposure to GEN. After exposure to 1-64 MIC of GEN for 24 hours, all strains developed resistance. This finding suggests that GEN monotherapy may not be an effective treatment choice although most CA-MRSAs are susceptible to GEN. Based on the in vitro data, optimum bactericidal activity of aminoglycosides is achieved when the exposure concentration is approximately 8-10 times the MIC against Gram negative organisms.13,14 In addition to maximal bactericidal activity, Blaser, et al. demonstrated that the peak/MIC ratio of 8 : 1 correlated with a decrease in the selection and re-growth of resistant subpopulations occurring during treatment with netilmicin.27 In contrast to other previous reports,6,25,26 our study had simulated the human pharmacokinetics, extended over a 48 hours period of evaluation and also identified the development of resistance in TD with IVPDM. During the TD dosing in YSSA1, resistance developed with re-growth of colony after 24 hours as in previous reports.6 However, when dosing was changed to OD, with a peak/MIC over 8.6 at least in both CA-MRSA strains, bactericidal activity was more profound after 24 hours time point and no re-growth or resistance occurred. One of the reasons would be the presence of longer duration of PAE in GEN OD regimen against CA-MRSA.14 Even though most peak/MIC data on aminoglycosides have been associated with Gram-negative bacteria, the results of our study suggest clues for maximizing their efficacies against CA-MRSA. We plan to extend the experiments to include efficacies and the development of resistance with antibiotic combination against MRSA.
Optimal therapy for S. aureus SCVs has not yet been defined.10 GEN is not generally recommended for SCVs because the interruption of electron transport reduces the electrochemical gradient across the bacterial membrane, resulting in a decreased uptake of GEN in addition to decreased susceptibility when compared to the parent strain.21 Baumert, et al. demonstrated the sensitivity of SCVs toward antibiotics known to be taken up through the electrical potential across the cytoplasma membrane (ΔΨ), such as aminoglycosides, dropped 10- to 30-fold when compared to the parent strain under routine MIC determination conditions.28 However, Park, et al. recently reported that OD dosing of arbekacin could reduce or eliminate the appearance of SCVs in IVPDM.19 In this study, SCVs appeared after 30 hours during TD treatment of GEN in YSSA15. They seemed to be selected by the slow-growing subpopulations. However, similar findings were not observed in OD. Gavalda, et al. showed that ceftriaxone plus gentamicin, both administered once a day, may be useful for selected cases of staphylococcal endocarditis.29 Further studies are warranted to determine whether OD or higher dose treatment could overcome the reduced uptake of GEN in SCVs of S. aureus, and to determine the beneficial period of the OD effects. This should be applied to the combination strategy with glycopeptide and aminoglycoside.
In conclusion, even if CA-MRSA is susceptible to GEN, traditional TD dosing could be less effective and induce resistance or SCVs during the 48 hours of the experiments. Although peak/MIC of GEN seemed not to be associated with better efficacies against GEN-susceptible CA-MRSA in the earlier period (- 8 hours), peak/MIC values more than 8.6 less from the OD regimen would have advantages of more killing effect with overcoming the emergence of resistance or SCVs of CA-MRSA during the later period (≥ 24 to 48 hours).
Figures and Tables
 | Fig. 2Time-kill curves of gentamicin against YSSA1 (A) and YSSA15 (B). The results are presented as means ± standard deviations (error bar) of colony counts (log10 CFU/mL) for measurements done in triplicate. GEN, gentamicin; q8h, every 8 hours; q24, every 24 hours; SCVs, small colony variants. |
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) KRF-2005-003-E00119.
References
1. Fridkin SK, Hagerman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005. 352:1436–1444.

2. Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005. 5:275–286.

4. Lee DG, Chun HS, Yim DS, Choi SM, Choi JH, Yoo JH, et al. Efficacies of vancomycin, arbekacin, and gentamicin alone or in combination against methicillin-resistant Staphylococcus aureus in an in vitro infective endocarditis model. Antimicrob Agents Chemother. 2003. 47:3768–3773.

5. Shelburne SA, Musher DM, Hulten K, Caesar H, Lu MY, Bhaila I, et al. In vitro killing of community-associated methcillin-resistant Staphylococcus aureus with drug combinations. Antimicrob Agents Chemother. 2004. 48:4016–4019.

6. Munckhof WJ, Kleinschmidt SL, Turnidge JD. Resistance development in community-acquired strains of methicillin-resistant Staphylococcus aureus: an in vitro study. Int J Antimicrob Agents. 2004. 24:605–608.

7. Rybak MJ, LaPlante KL. Community-associated methicillin-resistant Staphylococcus aureus: a review. Pharmacotherapy. 2005. 25:74–85.

8. Tsuji BT, Rybak MJ, Cheung CM, Amjad M, Kaatz GW. Community- and health care-associated methicillin-resistant Staphylococcus aureus: a comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn Microbiol Infect Dis. 2007. 58:41–47.

9. Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999. 43:1549–1555.
10. Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006. 4:295–305.
11. Schaaff F, Bierbaum G, Baumert N, Bartmann P, Sahl HG. Mutations are involved in emergence of aminoglycoside-induced small colony variants of Staphylococcus aureus. Int J Med Microbiol. 2003. 293:427–435.
12. Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006. 43:961–967.

13. Turnidge M. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2003. 17:503–528.

14. Kim M, Nicolau DP. Nightingale CH, Ambrose PG, Drusano GL, Murakawa T, editors. Antimicrobial pharmacodynamics in theory and clinical practice. 2007. 2nd ed. New York, NY: Informa Healthcare;147–175.
15. Park C, Lee DG, Kim SW, Choi SM, Park SH, Chun HS, et al. Predominance of community-associated methicillin-resistant Staphylococcus aureus strains carrying staphylococcal chromosome cassette mec type IVA in South Korea. J Clin Microbiol. 2007. 45:4021–4026.

16. Park C, Lee DG, Choi SM, Park SH, Choi JH, Yoo JH, et al. A case of perianal abscess due to Panton-Valentine leukocidin positive community-associated methicillin-resistant Staphylococcus aureus: report in Korea and literature review from the far east. Infect Chemother. 2008. 40:121–126.

17. CLSI performance standards for antimicrobial susceptibility testing; sixteenth informational supplement. 2006. Wayne, PA: Clinical Laboratory Standards Institute.
18. Bonapace CR, Friedrich LV, Bosso JA, White RL. Determination of antibiotic effect in an in vitro pharmacodynamic model: comparison with an established animal model of infection. Antimicrob Agents Chemother. 2002. 46:3574–3579.

19. Park YH, Lee DG, Chun HS, Park C, Park SH, Choi SM, et al. Once-daily dosing of arbekacin can suppress the formation of small colony variants of methicillin resistant Staphylococcus aureus in an in vitro pharmacodynamic infection model. Infect Chemother. 2006. 38:154–163.
20. Houlihan HH, Mercier RC, Rybak MJ. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methcillin-resistant Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997. 41:2497–2501.

21. von Eiff C, Peters G, Becker K. The small colony variant (SCV) concept -- the role of staphylococcal SCVs in persistent infections. Injury. 2006. 37:Suppl 2. S26–S33.
22. McGrath BJ, Lamp KC, Rybak MJ. Pharmacodynamic effects of extended dosing intervals of imipenem alone and in combination with amikacin against Pseudomonas aeruginosa in an in vitro model. Antimicrob Agents Chemother. 1993. 37:1931–1937.

23. Barclay ML, Begg EJ, Chambers ST. Adaptive resistance following single doses of gentamicin in a dynamic in vitro model. Antimicrob Agents Chemother. 1992. 36:1951–1957.
24. Domínguez MC, de La Rosa M, Borobio MV. Application of a spectrophotometric method for the determination of post-antibiotic effect and comparison with viable counts in agar. J Antimicrob Chemother. 2001. 47:391–398.

25. Schafer JA, Hovde LB, Rotschafer JC. Consistent rates of kill of Staphylococcus arueus by gentamicin over a 6-fold clinical concentration range in an in vitro pharmacodynamic model (IVPDM). J Antimicrob Chemother. 2006. 58:108–111.

26. Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006. 50:2626–2631.

27. Blaser J, Stone BB, Groner MC, Zinner SH. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987. 31:1054–1060.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download