Abstract
Purpose
In addition to cyclooxygenase-2 (COX-2) which is related to prostaglandin E2 synthesis, other enzymes such as cytosolic phospholipase A2 (cPLA2), microsomal prostaglandin E2 synthase-1 (mPGES-1), and 15-prostaglandin dehydrogenase (15-PGDH) have been suggested to be related to carcinogenesis of colorectal cancer (CRC). The aim of this study was to investigate the roles of cPLA2, COX-2, mPGES-1, and 15-PGDH in tumor progression.
Materials and Methods
cPLA2, COX-2, mPGES-1, 15-PGDH, and vascular endothelial growth factor (VEGF) expressions were immunohistochemically examined in 89 CRC, and their expressions were compared with each other or clinicopathologic parameters as well as VEGF as tumor progression parameters.
Results
cPLA2 was expressed in 54.5%, COX-2 in 80.5%, mPGES-1 in 96.4%, 15-PGDH in 46.1%, and VEGF in 65.9%. The expression of cPLA2 correlated with VEGF expression. COX-2 expression was correlated with the depth of invasion, tumor stage, cPLA2, and VEGF expressions. Moreover, VEGF revealed the highest expression in the tissues positive for both cPLA2 and COX-2. Furthermore, 15-PGDH expression was inversely correlated with VEGF expression.
Colorectal cancer (CRC) is one of the most common malignancies worldwide,1,2 and the adenoma-carcinoma sequence is a well-established aspect of its carcinogenesis.3 In addition to adenoma-carcinoma related genes, much attention has been focused on the involvement of cyclooxygenase (COX) in tumor development and progression.4 COX is a rate-limiting enzyme in the biosynthesis of prostaglandins from arachidonic acid, and exists in two well-characterized isoforms, i.e., COX-1 and COX-2. The former is constitutively expressed in a wide variety of tissues, where it serves a homeostatic function. On the other hand, COX-2 is not expressed under normal conditions, however, its expression is induced in response to cytokines, growth factors, tumor promoters, and others. Moreover, COX-2 expression has pathophysiologically been associated with inflammation, wound healing, and carcinogenesis. And high levels of constitutive COX-2 expression have been reported in many tumors, including those of the lung, breast, esophagus, stomach, and especially, in CRC. Furthermore, it has been suggested that COX-2 overexpression is associated with tumor formation and growth, angiogenesis, and metastasis.5-7 However, the clinical impact of COX-2 overexpression on oncologic outcomes in CRC is unclear. Some authors have concluded that COX-2 overexpression in CRC is associated with poor survival,8,9 whereas another study and our previous study failed to demonstrate that COX-2 overexpression in CRC is associated with a poor prognosis.10,11 Therefore, we hypothesized that COX-2-related oncologic effect could be influenced by other factors.
In addition to being dependent on COX-2, prostaglandin production is also directly dependent on the availability of free arachidonic acid (AA), which is released from membrane glycerophospholipid by the hydrolysis of fatty acid from its sn-2 position by PLA2.13 Several types of PLA2 are expressed in human cells, and 85 kDa IVA cytosolic PLA2 (cPLA2) of these is the major intracellular form. Moreover, cPLA2 is widely expressed and plays an essential role in stimulus-induced AA release.14,15 Because of its importance in the generation of prostaglands (PGs), it has also been suggested to participate in intestinal tumorigenesis.
PGH2, which is synthesized by the action of COX-2, is isomerized to PGE2 by terminal prostaglandin E synthase (PGES), and multiple isoforms of terminal PGES have so far been identified.12,16 The cytosolic PGES (cPGES) is constitutively expressed and functionally coupled to COX-1,17 whereas the microsomal prostaglandin E synthase-1 (mPGES-1) is rapidly induced by various stimuli in a COX-2 expression-related manner.12,16 The physiologic relevance of mPGES-1 expression in vivo has been substantiated recently by the finding that mPGES-1-deficient mice display attenuated responses to inflammation and pain.19 Furthermore, mPGES-1 is constitutively expressed in colon, lung, and gastric cancers,20-22 suggesting that it participates in tumorigenesis.
In addition, NAD+-linked 15-PGDH is of central importance during the biological inactivations of prostaglandins,23 and recent studies suggested that 15-PGDH has a tumor suppressing effect in several cancers.24,25
Thus, we undertook the present study to determine the effect of COX-2 and its coupled enzymes using human colorectal carcinoma samples. In addition to clinicopathologic parameters such as TNM staging, differentiation, and recurrence, we also evaluated VEGF expression, which is known to be up-regulated in most human tumors, including colorectal cancer, and has been associated with increased invasiveness, recurrent disease, and a poor prognosis.26 We then compared VEGF expression (which was used in this study as a tumor progression marker) with those of cPLA2, COX-2, mPGES-1, and 15-PGDH to determine whether these enzymes act as tumor promoters or suppressors.
Eighty-nine patients who had undergone surgical resection for primary sporadic colorectal carcinoma by a single colorectal surgeon at the Department of Surgery, Chosun University Hospital between March 2002 and December 2005 were included in this study. No patient had a history of hereditary CRC syndrome or regularly used aspirin-like drugs. Patients that received pre-operative chemotherapy or radiotherapy were not included. Included patients were followed up until death or August 2007; median post-operative follow up duration was 38.01 ± 12.58 months. Forty patients had colon cancer and 49 rectal cancer, and mean patient age was 63.43 ± 12.63 years. Samples were graded by pathologists according to the pathological features of tumors, i.e., histologic grade, lymph node metastasis, distant metastasis, and tumor stage (AJCC TNM classification). In all cases, archived H&E stained tissue slides were retrieved to confirm pathological features and to select suitable tissue blocks for immunohistochemical analysis.
A universal immunoenzyme polymer method was used for immunostaining. Four-µm thick sections were cut from formalin-fixed, paraffin-embedded tissue blocks, mounted on poly-lysine-coated slides, dewaxed in xylene, and rehydrated through a graded ethanol series. After deparaffinization, antigen retrieval treatment was performed at 121℃ (autoclave) for 15 minutes in 10 mmol/L sodium citrate buffer (pH 6.0), and sections were then treated with 3% hydrogen peroxide in methanol solution for 20 minutes in order to quench endogenous peroxidase activity. Nonspecific bindings were blocked by treating slides with Ultra V Block (UltraVision Plus Detection System, Thermo Scientific, Fremont, CA, USA) and incubation for 5 minutes at room temperature. The primary antibodies used were; mouse monoclonal antibody to cPLA2 (1 : 100, 4℃ overnight, Santa Cruz, CA, USA), mouse monoclonal antibody to COX-2 (1 : 400, 37℃ for one hour, Cayman, Ann Arbor, MI, USA), mouse monoclonal antibody to mPGES-1 (1 : 200, 37℃ for one hour, Cayman), rabbit polyclonal antibody to 15-PGDH (1 : 2,000, 4℃ overnight, Novus Biologicals, Littleton, CO, USA), and rabbit polyclonal antibody to VEGF (1 : 100, 37℃ for one hour, Santa Cruz). Each slide was washed four times in phosphate buffered saline (PBS). Biotinylated goat anti-mouse or goat anti-rabbit antibody (UltraVision Plus Detection System, Thermo Scientific, Fremont, CA, USA) was applied and incubated for 5 minutes at room temperature. Slides were washed again four times in PBS. Streptavidin-Alkaline Phosphate conjugate (UltraVision Plus Detection System) was then applied and incubated for 5 minutes at room temperature. Slides were washed four times in PBS. Fast Red/Naphtol Phosphate substrate was applied to the sections for visualization and incubated for 10-20 minutes. Sections were counterstained with Mayer's hematoxylin for 20 sec before air drying and coverslipping. Normal mouse serum IgG (Vector Laboratories, Burlingame, CA, USA) was used in place of the primary antibody as a negative control. Positive control tissue was colon cancers and inflammatory cells stained positively for VEGF, cPLA2, and mPGES-1. Normal colonic epithelium was used as internal control for PGDH. All experiments were performed in duplicate.
Each slide was assessed by a pathologist who was unaware of patient details. For cPLA2 and COX-2, the extent of staining was graded as follows: 0 - staining in less than 1% of tumor cells; 1 - staining in 1-20%; 2 - staining in 20-50%; and 3 - staining in more than 50%. Overall intensity of staining was also assessed as follows: 0 no staining; 1 weak staining; 2 moderate staining; and 3 strong staining. Final scores (range from 0 to 9) were obtained by multiplying staining extents and intensities. Final scores were described as follows: 0, no expression; 1 to 3, weak expression; 4-6, moderate expression; and 7-9, strong expression.27 For statistical analysis, no expression and weak expression were combined and described as negative for expression, and moderate and strong expression were combined and described as positive for expression.
In the case of mPGES-1, percentages of tumor cells showing cytoplasmic staining were scored as follows; moderate to strong cytoplasmic positivity in > 10% of tumor cells was regarded as positive, and faint cytoplasmic staining or moderate to strong cytoplasmic expression observed in < 10% of tumor cells was regarded as negative.28 For 15-PGDH, samples were defined as positive when ≥ 30% of tumor cells were stained, and as negative when < 30% of tumor cells were stained.29
The χ2 test and Fisher's exact probability were used to analyze the relationship of cPLA2, COX-2, mPGES-1 and 15-PGDH according to clinicopatologic parameters or VEGF expression. Significance was defined as p values of < 0.05. The SPSS version 12.0 software (SPSS Inc., Chicago, IL, USA) package was used for statistical analysis.
cPLA2 staining was observed in the smooth muscle of the muscularis propria and the muscularis mucosa, in some superficial interstitial cells, and in rare endothelial cells. In normal epithelial cells, there was no staining or weak cPLA2 staining. COX-2 staining revealed negligible expression in normal epithelium, but districtly positive staining in the interstitial cells, whereas mPGES-1 was not expressed in the adjacent normal mucosal epithelial cells. In normal mucosa, 15-PGDH was seen in epithelial cells forming villi (Fig. 1).
cPLA2 expression was positive in 54.4% (48/88) of tumor samples, COX-2 in 80.5% (66/82), mPGES-1 in 96.4% (81/84), and 15-PGDH in 46.1% (41/89). cPLA2 expression was significantly related to VEGF expression (p = 0.016). Although cPLA2 expression was not significantly related to clinicopathologic parameters such as TNM staging, lymph node status, grade of differentiation, and recurrence, cPLA2 expression tended to be high in patients with an advanced stage or unfavorable clinicopathologic features such as pT3/pT4, positive lymph node metastasis, elevated pre-operative CEA levels, and positive recurrence. COX-2 expression was correlated with depth of invasion (p = 0.002), TNM stage (p = 0.011), and VEGF expression (p < 0.001). mPGES-1 was expressed in most of the samples studied, however, its expression was not found to be related to clinicopathologic parameters. 15-PGDH expression was inversely correlated with VEGF expression (p = 0.040). The expression of 15-PGDH was high in patients with earlier stage disease or favorable clinicopathologic features, such as well-differentiated cancer, normal pre-operative carcinoembryonic levels, and no tumor recurrence. However, these relations were not statistically significant.
Seventy nine of total 89 cases were evaluated for both COX-2 expression and cPLA2 expression. Comparing the correlation of their expression with their staining scores, COX-2 expression was positively correlated with cPLA2 expression (Fig. 2, p = 0.002), but not with 15-PGDH expression (data not shown).
VEGF expression was positive in 65.9% of tissue samples, but didn't stain in the adjacent normal mucosal epithelium. To clarify whether cPLA2 expression augments the oncogenic effect of COX-2, we grouped the expression into 4 categories, i.e., group I, cPLA2 (-) and COX-2 (-); group II, cPLA2 (+) and COX-2 (-); group III, cPLA2 (-) and COX-2 (+); and group IV cPLA2 (+) and COX-2 (+). These groups were then compared with the status of VEGF expression. VEGF expression was positive in 20.0% of group I, in 33% of group II, in 61.5% of group III, and 83.3% in group IV, showing the highest expression rate in both cPLA2 and COX-2 positive cases. cPLA2 expression was found to be a significant marker of VEGF expression in addition to COX-2 expression (Table 2, p = 0.001).
In the present study, we evaluated the expression profiles of cPLA2, COX-2, mPGES-1, and 15-PGDH in patients with colorectal cancer. The expressions of these entities are known to be related closely to the synthesis and degradation of PGE2, which is known to be associated with resistance to programmed cell death, cell migration, cell proliferation, and angiogenesis. However, there is so far no study evaluating all these enzymes together. In the present study, we demonstrated that cPLA2, COX-2, and mPGES-1 were overexpressed in CRC tissues, and that cPLA2 and COX-2 overexpressions were correlated with tumor progression. Furthermore, 15-PGDH expression was inversely correlated with tumor progression.
In this study, we found that COX-2 was overexpressed in 80.5% of tissue samples tested, and that COX-2 expression was closely related to T-stage, TNM stage, and VEGF expression status, suggesting that COX-2 participates in tumor progression. These findings are in good agreement with those of other studies.8,9 In our previous studies, we used a similar scoring system of COX-2, and found that COX-2 was expressed in 42.4% to 47.8% of tumor tissues, proven immunohistochemically,11,31 which is quite different from that found in the present result. However, the two studies differ in terms of the secondary antibody kit used (although the same primary antibody was used).
It is unclear to what extent COX-2 overexpression influences tumor progression, or whether it has a prognostic impact in CRC. A number of studies found that COX-2 expression is a useful prognostic marker in CRC,8,9 whereas others, including our previous study, demonstrated that its overexpression has little prognostic impact, although COX-2 is aberrantly overexpressed in CRC.10,11,32,33 Thus, the present study was designed to identify whether or not the enzymes involved in the metabolism of PGE2 affected tumor progression.
Studies on the profiles and effects of cPLA2 expression in CRC have also revealed disparate results. In two animal models of familial adenomatous polyposis, cPLA2 was overexpressed in small bowel adenoma, but not in colonic adenoma. Furthermore, cPLA2 knock-out mice were found to have dramatically reduced small bowel polyp development but not colonic polyp development, suggesting that cPLA2 had an oncologic effect.34,35 On the other hand, cPLA2 expression was diminished in azoxymethane-induced mouse colon tumors,36 and knock-out of cPLA2 enhanced colon tumor development.37 Moreover, available results on cPLA2 expression in human colorectal adenocarcinoma have also been highly variable. Some have suggested that cPLA2 is overexpressed,38-40 whereas others have concluded to the contrary.36,41,42 However, the study sample sizes were relatively small, i.e., ranging from 5 to 48. Panel, et al.27 reported that cPLA2 was overexpressed in 49% of 65 patients with colorectal cancers, and that cPLA2 expression was correlated with COX-2 expression, which concurs with our findings; i.e., we demonstrated that cPLA2 was overexpressed in 54.5% of CRCs (n = 88), and that cPLA2 expression was correlated with the expressions of COX-2 and VEGF. Furthermore VEGF levels were found to be significantly elevated when both cPLA2 and COX-2 were expressed. These results strongly suggest that cPLA2 plays an important role in tumor development and/or progression in CRC, and that the expressions of cPLA2 plus COX-2 might be a good marker of tumor progression and/or prognosis.
Like COX-2, it has been reported that mPGES-1 is constitutively overexpressed in many cancers, including colorectal cancer.21,22,28,43 Yoshimatsu, et al.43 reported that mPGES-1 was overexpressed in more than 80% of human colorectal cancers and adenomas. We also demonstrated that mPGES-1 was expressed in most invasive colorectal cancers and in carcinoma in situ. It has been suggested that aberrant mPGES-1 expression in combination with COX-2 expression could contribute to tumorigenesis in vitro.20 And Nakanishi, et al.44 reported that genetic deletion of mPGES-1 suppressed intestinal tumorigenesis, and suggested that mPGES-1 could be a target for cancer chemoprevention with the potential for improved tolerability over traditional nonsteroidal anti-inflammatory drugs and selective COX-2 inhibitors. mPGES-1 is specifically involved in PGE2 synthesis, but not in synthesis of other prostaglandins such as PGI2 decrease of which is responsible for side effect of long-term use of NSAID or COX-2 inhibitors. Furthermore, mPGES-1 is constitutively overexpressed at all stages of tumors. Therefore, it may be a attractive therapeutic target in terms of chemoprevention and overcoming the shortcomings of COX-2 inhibitors.
Most of the PGE2 synthesized by cPLA2, COX-2, and mPGES-1 are degraded by 15-PGDH, which catalyzes the NAD+-dependent oxidation of the 15-OH group of prostaglandin to a 15-keto group.23 Accordingly, 15-PGDH is a normal physiologic antagonist of COX-2. Yan, et al.45 found that 15-PGDH mRNA and protein are highly expressed in normal colonic epithelial tissues, but almost undetectable in colon cancer tissues, and that the restoration of 15-PGDH levels inhibit tumor formation in mice, thus providing strong evidence that 15-PGDH could act as a tumor suppressor during colon cancer development. However, the expression of 15-PGDH in human cancer tissues has only been studied in a small number of cancers and its expression has been found to vary widely. Yan, et al. reported that 15-PGDH expression was immunohistochemically negative in 16 of 17 colon cancer cases and in 10 of 13 gastric cancers. In one study using Northern and Western blotting-based assay, 15-PGDH levels were lower in 85% of 23 pairs of colon carcinoma samples than in adjacent normal mucosa,24 and in another study using real time RT-PCR study in breast cancer, 15-PGDH expression was down-regulated in 64% (16 of 28) of tumors.46 In addition, one bladder cancer study found that 15-PGDH was expressed in 64% (18 of 28) of transitional cell carcinomas and in 1 of 10 squamous cell carcinomas.47 In contrast, however, Yoo, et al.29 reported that 15-PGDH was immunohistochemically expressed in 54 (90%) of 60 in gastric cancer. In the present study, 15-PGDH was found to be expressed in 46.1% (41 of 89) of colorectal cancers, and its expression showed a trend of positive expression in patients with favorable clinicopathologic parameters, such as pT0, well differentiated histologic pattern, normal pre-operative carcinoembryonic antigen levels, and no recurrence (Table 1). Furthermore, we found that 15-PGDH expression was not correlated with COX-2 expression, and that its expression was significantly higher in tumor tissues which did not express VEGF. Our results suggest that 15-PGDH has tumor suppressive activity in colorectal cancer, However, further large scaled studies are needed to clarify the precise role of 15-PGDH played in tumor progression.
In conclusion, the present study demonstrates that cPLA2 and mPGES-1, in addition to COX-2, are constitutively overexpressed, and that 15-PGDH might be attenuated in colorectal carcinoma. Furthermore, cPLA2 and 15-PGDH as well as COX-2 could have an important role in tumor progression.
Figures and Tables
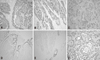 | Fig. 1Immunohistochemical staining. (A) positive cPLA2 in tumor tissue. (B) Positive COX-2 in tumor tissue. (C) Positive mPGES-2 in tumor tissue. (D) Positive 15-PGDH in normal colonic epithelium. (E) Negative 15-PGDH in tumor tissue. (F) Positive VEGF in tumor tissue. cPLA2, cytosolic phospholipase A2; COX-2, cyclooxygenase 2; mPGES-2, microsomal prostaglandin E synthase-2; PGDH, prostaglandin dehydrogenase; VEGF, vascular endothelial growth factor. |
 | Fig. 2Relationship between COX-2 and cPLA2 expression in colorectal cancer (n = 79, r = 0.339, p = 0.002). COX-2, cyclooxygenase 2; cPLA2, cytosolic phospholipase A2. |
Table 1
Correlation of Clinicopathologic Parameters with cPLA2, COX-2, mPGES-1, and 15-PGDH Expressions
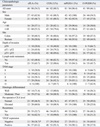
cPLA2, cytosolic phospholipase A2; COX-2, cyclooxygenase 2; mPGES-1, microsomal prostaglandin E2 synthase-1; 15-PGDH, 15-prostaglandin dehydrogenase; CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor.
*Negative VEGF expression vs. positive VEGF expression: p = 0.016.
†pT0 vs. pT1/pT2 vs. pT3/pT4: p = 0.002.
‡TNM stage 0 vs. I vs. II vs. III vs. IV p = 0.011.
§Negative VEGF expression vs. Positive VEGF expression: p < 0.001.
∥Negative VEGF expression vs. Positive VEGF expression: p = 0.040.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Clinical Medicine Research Institute of Chosun University Hospital (2006).
References
1. Boyle P, Zaridze DG, Smans M. Descriptive epidemiology of colorectal cancer. Int J Cancer. 1985. 36:9–18.
2. Annual Report of the Korea Central Cancer Registry. 2002. Ministry of Health and Welfare Republic of Korea.
4. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999. 18:7908–7916.

5. Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000. 37:431–502.

6. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003. 24:96–102.

7. Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ. Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem. 1999. 274:10911–10915.

8. Yamauchi T, Watanabe M, Kubota T, Hasegawa H, Ishii Y, Endo T, et al. Cyclooxygenase-2 expression as a new marker for patients with colorectal cancer. Dis Colon Rectum. 2002. 45:98–103.

9. Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004. 10:8465–8471.
10. Fux R, Schwab M, Thon KP, Gleiter CH, Fritz P. Cyclooxygenase-2 expression in human colorectal cancer is unrelated to overall patient survival. Clin Cancer Res. 2005. 11:4754–4760.

11. Lim SC, Lee TB, Choi CH, Ryu SY, Min YD, Kim KJ. Prognostic significance of cyclooxygenase-2 expression and nuclear p53 accumulation in patients with colorectal cancer. J Surg Oncol. 2008. 97:51–56.

12. Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Ann Rev Biochem. 1986. 55:69–102.

13. Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999. 39:175–189.
14. Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997. 272:16709–16712.

15. Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997. 390:622–625.

16. Forsberg L, Leeb L, Thorén S, Morgenstern R, Jakobsson P. Human glutathione dependent prostaglandin E synthase: gene structure and regulation. FEBS Lett. 2000. 471:78–82.

17. Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000. 275:32775–32782.

18. Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000. 275:32783–32792.

19. Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003. 100:9044–9049.
20. Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J Biol Chem. 2003. 278:19396–19405.

21. Yoshimatsu K, Altorki NK, Golijanin D, Zhang F, Jakobsson PJ, Dannenberg AJ, et al. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001. 7:2669–2674.
22. van Rees BP, Sivula A, Thorén S, Yokozaki H, Jakobsson PJ, Offerhaus GJ, et al. Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer. 2003. 107:551–556.

23. Ensor CM, Tai HH. 15-Hydroxyprostaglandin dehydrogenase. J Lipid Mediat Cell Signal. 1995. 12:313–319.
24. Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005. 280:3217–3223.

25. Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006. 103:12098–12102.

26. Thornton AD, Ravn P, Winslet M, Chester K. Angiogenesis inhibition with bevacizumab and the surgical management of colorectal cancer. Br J Surg. 2006. 93:1456–1463.

27. Panel V, Boëlle PY, Ayala-Sanmartin J, Jouniaux AM, Hamelin R, Masliah J, et al. Cytoplasmic phospholipase A2 expression in human colon adenocarcinoma is correlated with cyclooxygenase-2 expression and contributes to prostaglandin E2 production. Cancer Lett. 2006. 243:255–263.
28. Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006. 208:356–363.

29. Yoo NJ, Jeong EG, Lee SH, Lee SH. Expression of 15-hydroxyprostaglandin dehydrogenase, a COX-2 antagonist and tumour suppressor, is not altered in gastric carcinomas. Pathology. 2007. 39:174–175.

30. Choi JY, Jang KT, Shim YM, Kim K, Ahn G, Lee KH, et al. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol. 2006. 13:1054–1062.

31. Lim SC, Lee TB, Choi CH, Ryu SY, Kim KJ, Min YD. Expression of cyclooxygenase-2 and its relationship to p53 accumulation in colorectal cancers. Yonsei Med J. 2007. 48:495–501.
32. Petersen S, Haroske G, Hellmich G, Ludwig K, Petersen C, Eicheler W. COX-2 expression in rectal carcinoma: immunohistochemical pattern and clinical outcome. Anticancer Res. 2002. 22:1225–1230.
33. Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002. 97:1037–1041.
34. Takaku K, Sonoshita M, Sasaki N, Uozumi N, Doi Y, Shimizu T, et al. Suppression of intestinal polyposis in Apc(delta 716) knockout mice by an additional mutation in the cytosolic phospholipase A(2) gene. J Biol Chem. 2000. 275:34013–34016.

35. Hong KH, Bonventre JC, O'Leary E, Bonventre JV, Lander ES. Deletion of cytosolic phosholipase A(2) suppresses Apc(Min)-induced tumorigenesis. Proc Natl Acad Sci U S A. 2001. 98:3935–3939.

36. Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003. 24:307–315.

37. Ilsley JN, Nakanishi M, Flynn C, Belinsky GS, De Guise S, Adib JN, et al. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005. 65:2636–2643.

38. Soydan AS, Tavares IA, Weech PK, Temblay NM, Bennett A. High molecular weight phospholipase A2 and fatty acids in human colon tumors and associated normal tissue. Eur J Cancer. 1996. 32A:1781–1787.
39. Dimberg J, Samuelsson A, Hugander A, Söderkvist P. Gene expression of cyclooxygenase-2, group II and cytosolic phospholipase A2 in human colorectal cancer. Anticancer Res. 1998. 18:3283–3287.
40. Wendum D, Svrcek M, Rigau V, Boëlle PY, Sebbagh N, Parc R, et al. COX-2, inflammatory secreted PLA2, and cytoplasmic PLA2 protein expression in small bowel adenocarcinoma compared with colorectal adenocarcinomas. Mod Pathol. 2003. 16:130–136.

41. Dong M, Johnson M, Rezaie A, Ilsley JN, Nakanishi M, Sanders MM, et al. Cytoplasmic phospholipase A2 levels correlate with apoptosis in human colon tumorigenesis. Clin Cancer Res. 2005. 11:2265–2271.

42. Laye JP, Gill JH. Phospholipase A2 expression in tumors: a target for therapeutic intervention? Drug Discov Today. 2003. 8:710–716.

43. Yoshimatsu K, Golijanin D, Paty PB, Soslow RA, Jakobsson PJ, DeLellis RA, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenoma and cancer. Clin Cancer Res. 2001. 7:3971–3976.
44. Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008. 68:3251–3259.

45. Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004. 101:17468–17473.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download