Abstract
Purpose
Mitochondrial encephalopathy (ME) is a rare disorder of energy metabolism. The disease course can roughly be evaluated by clinical findings. The purpose of this study was to evaluate metabolic spectral changes using proton MR spectroscopy (MRS), and to establish a way to monitor ME by neuroimaging.
Materials and Methods
Proton MRS data were retrospectively reviewed in 12 patients with muscle biopsy-confirmed ME (M : F = 7 : 5, Mean age = 4.8 years). All received 1H-MRS initially and also after a ketogenic diet and mitochondrial disease treatment cocktail (follow up average was 10.2 months). Changes of N-acetylaspartate/creatine (NAA/Cr) ratio, choline/creatine (Cho/Cr) ratio, and lactate peak in basal ganglia at 1.2 ppm were evaluated before and after treatment. Findings on conventional T2 weighted MR images were also evaluated.
Results
On conventional MRI, increased basal ganglia T2 signal intensity was the most common finding with ME (n = 9, 75%), followed by diffuse cerebral atrophy (n = 8, 67%), T2 hyperintense lesions at pons and midbrain (n = 4, 33%), and brain atrophy (n = 2, 17%). Lactate peak was found in 4 patients; 2 had disappearance of the peak on follow up MRS. Quantitative analysis showed relative decrease of Cho/Cr ratio on follow up MRS (p = 0.0058, paired t-test, two-tailed). There was no significant change in NAA/Cr ratio.
Mitochondrial encephalopathy (ME) is a heterogeneous group of clinical syndromes associated with mitochondrial energy metabolism abnormalities. Presentation is nonspecific with encephalomyopathy, failure to thrive, seizures, ophthalmoplegia, and sensorineural hearing loss.1,2 Impaired energy production results from overall dysfunction of the mitochondrial respiratory chain, which is composed of five enzymatic complexes embedded in the inner mitochondrial membrane.
There is no specific treatment for ME, and only conservative care is available. One treatment is ketogenic diet therapy with a mitochondrial disease treatment cocktail of coenzyme Q10, riboflavin, L-carnitine, and high-dose multivitamins; some favorable results have been reported.3,4
Diagnosis and monitoring can be achieved by clinical findings and imaging techniques such as CT, conventional MR imaging, and diffusion weighted MRI.5-7 MR spectroscopy (MRS) is a useful tool in evaluation of brain tissue metabolites; applications in mitochondrial encephalopathy are well documented.8-13 However, previous studies have been limited to a single MRS study; there are no reports on serial metabolite distribution changes during treatment. The purpose of this study is to evaluate changes in the metabolic spectrum by MRS spectroscopy, and to establish a way to monitor disease course by neuroimaging.
The institutional review board approved retrospective analysis of MRS data from patients with mitochondrial encephalopathy. From July 2005 to October 2007, there were 46 patients with muscle biopsy-confirmed mitochondrial disease. Twelve received 1H-MRS (M : F = 7 : 5, Mean age = 4.8 years, range from 1 to 17 years) initially and also after ketogenic diet and mitochondrial disease treatment cocktail administration. The average interval between MRS scans was 10.2 months (range 5 to 17 months). Data were retrospectively reviewed.
Localized 1H-MRS was performed on a 3.0T MRI system (Achieva, Philips Medical Systems, Best, Netherlands) using an 8-channel SENSE head coil. Two water-suppressed 1H-MR spectra were obtained from a voxel located in the basal ganglia (voxel, 2 × 2 × 2 mL). The following spectral acquisition parameters were used with the point resolved spectroscopy sequence (PRESS) method: Spectra BW = 2,000 Hz, TR = 2.0 s, TE = 288/144 ms, acquisition number = 128. All raw data were processed using the SpectroView software package (Philips Medical System, Best, Netherlands), with Gaussian line broadening of 3Hz, zero, and linear phase correction. Peaks were identified with known chemical shifts: lactate at 1.2 ppm, N-acetylaspartate at 2.02 ppm, phosphocreatinine and creatinine at 3.05 ppm, and choline-containing compounds at 3.22 ppm. Pre- and post-treatment MR spectra were evaluated for change in choline/creatinine (Cho/Cr) and N-acetylasparte/creatinine (NAA/Cr) ratios. Presence of a lactate doublet peak was also monitored at 1.2 ppm, and confirmed by showing inversion on half TE sequence (TE = 144 ms) [(-): absence of lactate doublet, (+): faint presence of lactate doublet, (++): definite presence of lactate doublet]. Morphological findings on conventional T2 weighted images were also assessed, with emphasis on abnormal signals in the basal ganglia, brain stem, and any atrophic changes in brain parenchyma.
Six, five, and one patient were diagnosed with mitochondrial respiratory chain (MRC) I, MRC IV, and MRC II deficiency, respectively (Table 1). Clinically, 2 (patients No. 2 and 11) had Leigh disease and 1 (patient No. 8) had Kearn-Sayre Syndrome. The other 9 had uncategorized MRC encephalopathies. All had mild to moderate clinical improvement between pre- and post-treatment MRS studies, such as decreased seizure frequency, improved general condition, and improved developmental status.
On conventional T2 weighted images (T2WI), all had abnormal findings. Increased basal ganglia T2 signal intensity was the most common finding with ME (n = 9, 75%), followed by diffuse cerebral atrophy (n = 8, 67%). Four (33%) patients had increased midbrain and pons signal lesions. Cerebellar atrophy (17%) was found in 2 patients, along with diffuse cerebral atrophy (Table 1). Lactate peak was found in 4 patients; 2 had disappearance of the peak on follow up scan. One patient showed marked reduction of lactate peak after ketogenic diet and mitochondrial cocktail therapy (Case #2, Fig. 1), but the other patient showed no remarkable change of lactate peak before and after treatment.
Quantitative analysis showed relative decrease of Cho/Cr ratio on follow up MRS (p = 0.0058, paired t-test, two-tailed). There was no significant change in NAA/Cr ratio. However, all three patients with normal basal ganglia signal on T2WI showed elevated NAA/Cr ratio after ketogenic diet. On anatomical imaging, there were no remarkable changes of brain atrophy or newly developed T2 hyperintense lesions.
Mitochondrial respiratory chain disease or encephalopathy is a rare disorder of energy metabolism. Clinical characteristics and prognosis are still under investigation. With the development of electron microscopic diagnosis and enzyme assay technique, many children with previous uncertain etiologies have been diagnosed with ME. Typical imaging findings are non-specific cortical T2 hyperintensities, progressive atrophy, and encephalitis, similar to findings without evidence of infectious etiologies.
Using brain MR examinations, we investigated 12 patients with established ME, and found a wide range of structural and metabolic abnormalities. Our results are in agreement with previous studies which support the role of MRS as a confirmatory test.1,11,12,14,15 Our study also supports the use of MRS for monitoring ME treatment by measuring the Cho/Cr ratio and lactate peak. As clinical symptoms and neurologic function improve, the Cho/Cr ratio and lactate amplitude decrease.
As MRS can detect biochemical metabolites concentration in vivo, it is more sensitive than MR imaging in assessment of brain tissue metabolite alterations, such as increased lactate fraction. In our study, all patients had various T2WI MR findings which did not change during treatment, while differences in metabolite fraction were seen on serial MRS.
Although there are doubts about ketogenic diet efficiency,16 recent studies present remarkable successes. Prasad, et al.17 showed that in intractable epilepsy treated with ketogenic diet, nearly one third became seizure-free, another third had significantly reduced seizure frequency, and the remainder did not benefit significantly. Our patients had no remarkable complications with conservative treatment. Instead, seizure frequency was decreased and neurologic function improved. These clinical findings were correlated well with MRS changes.
This study has some limitations. First, there are no age matched controls for pediatric MRS data. We could only compare two consecutive studies with longitudinal analysis. Previous MRS studies of healthy children have revealed that basal ganglia NAA increases until adolescence.18 Therefore, NAA increase during ME disease course cannot be explained by treatment effects. Also, Gupta, et al.19 showed that adequate treatment for hypoparathyroidism induced gradual Cho/Cr reduction, similar to our study. As choline decreases according to myelination and maturation, Cho/Cr reduction is not affected solely by a ketogenic diet. However, lactate peak is definitely abnormal in disease conditions, such as ME, therefore, reduction or resolution is a good indicator for disease monitoring or treatment assessment.
Second, this study is a retrospective review of MRS data in a large patient pool, therefore, follow up periods are quite variable. Ideal and future study design should include pre- and post-treatment serum and CSF lactate level check, uniform follow up periods and more homogenous patient group selection. Definition of mitochondrial encephalopathy is still under investigation, therefore, we included wide variety of diseases, however, future application should be focused on more specific disease group. Third, as there is no specific treatment for ME, multiple therapies, such as antiepileptics, ketogenic diets, and mitochondrial disease cocktails, were used. It is, therefore, difficult to identify treatments responsible for clinical improvement and MRS changes.
In short, this is a primitive and pilot study of MR spectroscopy uses in mitochondrial disease. Diverse patient characteristics and follow up periods are basic limitations of this study. Furthermore, obtaining normal controls for MR spectroscopy is necessary. Data from normal children and sham control are basically impossible due to disease nature and ethical problems. However, this study has clarified some of the diverse findings of ME on routine MRI & MR spectroscopy; these results can provide benifits to future research.
In conclusion, we found that MRS is a useful tool for monitoring disease progression or improvement in ME. Despite lack of change on T2 weighted images, MRS depicted metabolite changes during ketogenic diet and mitochondrial cocktail therapy. Decreased lactate peak and Cho/Cr fraction were correlated well with improvement of clinical symptoms.
Figures and Tables
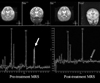 | Fig. 1Two-year-old male with Leigh disease (case 2). Pre-treatment MRS shows high lactate peak (arrow) at basal ganglia. One year follow up MRS shows decrease of lactate (double arrows) and restoration of NAA. NAA, N-acetylaspartate. |
References
2. Chinnery PF, Turnbull DM. Clinical features, investigation, and management of patients with defects of mitochondrial DNA. J Neurol Neurosurg Psychiatry. 1997. 63:559–563.

3. Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007. 48:82–88.
4. Kang HC, Kim HD, Lee YM, Han SH. Landau-Kleffner syndrome with mitochondrial respiratory chain-complex I deficiency. Pediatr Neurol. 2006. 35:158–161.

5. Yonemura K, Hasegawa Y, Kimura K, Minematsu K, Yamaguchi T. Diffusion-weighted MR imaging in a case of mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. AJNR Am J Neuroradiol. 2001. 22:269–272.
6. Clark JM, Marks MP, Adalsteinsson E, Spielman DM, Shuster D, Horoupian D, et al. MELAS: Clinical and pathologic correlations with MRI, xenon/CT, and MR spectroscopy. Neurology. 1996. 46:223–227.
7. Barragán-Campos HM, Vallée JN, Lô D, Barrera-Ramírez CF, Argote-Greene M, Sánchez-Guerrero J, et al. Brain magnetic resonance imaging findings in patients with mitochondrial cytopathies. Arch Neurol. 2005. 62:737–742.

8. Bianchi MC, Tosetti M, Battini R, Manca ML, Mancuso M, Cioni G, et al. Proton MR spectroscopy of mitochondrial diseases: analysis of brain metabolic abnormalities and their possible diagnostic relevance. AJNR Am J Neuroradiol. 2003. 24:1958–1966.
9. Flemming K, Ulmer S, Duisberg B, Hahn A, Jansen O. MR spectroscopic findings in a case of Alpers-Huttenlocher syndrome. AJNR Am J Neuroradiol. 2002. 23:1421–1423.
10. Pavlakis SG, Kingsley PB, Kaplan GP, Stacpoole PW, O'Shea M, Lustbader D. Magnetic resonance spectroscopy: use in monitoring MELAS treatment. Arch Neurol. 1998. 55:849–852.
11. Salvan A, Vion-Dury J, Confort-Gouny S, Sangla I, Pouget J, Cozzone PJ. Brain metabolic profiles obtained by proton MRS in two forms of mitochondriopathies: Leber's hereditary optic neuropathy and chronic progressive external ophthalmoplegia. Eur Neurol. 1998. 40:46–49.

12. Möller HE, Wiedermann D, Kurlemann G, Hilbich T, Schuierer G. Application of NMR spectroscopy to monitoring MELAS treatment: a case report. Muscle Nerve. 2002. 25:593–600.

13. Kuhl CK, Layer G, Träber F, Zierz S, Block W, Reiser M. Mitochondrial encephalomyopathy: correlation of P-31 exercise MR spectroscopy with clinical findings. Radiology. 1994. 192:223–230.

14. Kolb SJ, Costello F, Lee AG, White M, Wong S, Schwartz ED, et al. Distinguishing ischemic stroke from the stroke-like lesions of MELAS using apparent diffusion coefficient mapping. J Neurol Sci. 2003. 216:11–15.

15. Kim HS, Kim DI, Lee BI, Jeong EK, Choi C, Lee JD, et al. Diffusion-weighted image and MR spectroscopic analysis of a case of MELAS with repeated attacks. Yonsei Med J. 2001. 42:128–133.

16. Erickson JC, Jabbari B, Difazio MP. Basal ganglia injury as a complication of the ketogenic diet. Mov Disord. 2003. 18:448–451.

17. Prasad AN, Stafstrom CF, Holmes GL. Alternative epilepsy therapies: the ketogenic diet, immunoglobulins, and steroids. Epilepsia. 1996. 37:Suppl 1. S81–S95.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download