Abstract
Purpose
The purpose of this study is to explore the dynamic change of brain-derived neurotrophic factor (BDNF) mRNA, protein, and tyrosine kinase-coupled receptor (TrkB) mRNA of the rat hippocampus under different stress conditions and to explore the influence of senescence on the productions expression.
Materials and Methods
By using forced-swimming in 4℃ cold ice water and 25℃ warm water, young and aged male rats were randomly divided into acute stress (AS) and chronic mild repeated stress (CMRS) subgroups, respectively. BDNF productions and TrkB mRNA in the hippocampus were detected by using Western-blotting and reverse transcription-polymerase chain reaction (RT-PCR), separately, at 15, 30, 60, 180, and 720 min after the last stress session.
Results
The short AS induced a significant increase in BDNF mRNA and protein in both age groups, but the changes in the young group were substantially greater than those of the aged group (p < 0.005). The CMRS resulted in a decrease in BDNF mRNA and protein, but a significant increase in TrkB mRNA in both young and age groups. The expression of BDNF mRNA and protein in the AS groups were higher than in the CMRS groups at 15, 30, and 60 min after stress.
Brain-derived neurotrophic factor (BDNF) is directly involved in neurite outgrowth and regulates the survival, differentiation, and maintenance of function in different neuronal populations.1 Tyrosine kinase-coupled receptor (TrkB) is the primary signal transduction receptor for BDNF. BDNF is the central neurotrophic factor (NTF) for neurons and neurogliocytes, and plays an important role in hippocampal aging. In fact, BDNF and TrkB expression have been shown to decrease both in the normal aging hippocampus and in the Alzheimer's patient hippocampus, while they significantly increase during learning-related events.2-5
The hippocampus is one of the important brain areas that is connected with learning and memory, and it is also an area that is very susceptible to stress and senescence.6,7 It is well-established that stress is a conspicuous factor of neuronal injury and can trigger degenerative cellular processes in the limbic system.8,9 Single or repeated immobilization stress treatments have been reported to decrease BDNF mRNA throughout the hippocampus.10-12 In contrast, BDNF mRNA expression measured by in situ hybridization increased as early as 15 min in most hippocampal regions and was significantly augmented after 180 min of stress exposure. Adlard reported that the expression of BDNF obviously decreased at time-course 5 and 10 hour after binding stress, but the expression of TrkB mRNA showed no change in dentate gyrus and hippocampus after single stress.9
Although it is recognized that BDNF is the most important NTF in critical CNS functions, including neuronal development and differentiation, time-course studies of different stress applications have not yet been investigated in terms of aging responses. In the present study, we investigated whether both acute stress (AS) and repeated chronic mild stress applications might modify expression of hippocampal BDNF mRNA and protein and TrkB mRNA content, or whether there was an age-related change in BDNF expression in the hippocampus of rats.
Animal behaviors including attacking behavior, languishing behavior, and excreting behavior evidently change in stressful conditions. Aging is an important factor affecting the behavior of animals and human being. The exploratory behaviors and locomotor activities during open field (OF) test can reveal the rat's adaptation to a new environment,13,14 which indicates the cognitive ability of rats.
Therefore, in order to clarify the changes in BDNF after different stresses and the effect of aging on the expression of BDNF protein, we studied the expression of BDNF mRNA in the hippocampus following forced-swimming stress by reverse transcription-polymerase chain reaction (RT-PCR) with an endogenous internal standard, and detected the expression of BDNF protein by Western blotting.
Male Sprague-Dawley rats aged 2 months and 22 months were obtained from the Animal Experiment Center of Binzhou Medical University, and housed for 1 week prior to the experiment under a constant temperature (21 ± 1℃) and lighting regimen (light on from 7:00 am to 7:00 pm). Food pellets and water were available ad libitum throughout the experiments. Young and aged rats were randomly divided into three subgroups: the AS group (n = 30), the chronic mild repeated stress (CMRS) group (n = 30), and a control group (n = 8).
AS model: animals were forced to constantly swim in 4℃ water in a 70×40×80 cm aquarium (35 cm depth of water) for 10 minutes on the day of the experiment (Fig. 1A). CMRS model: animals were forced to swim in 25℃ water for 10 minutes at 8:30 am, which was enforced for 21 consecutive days in the same aquarium (Fig. 1B). After the conclusion of the different stress tests, animals were immediately decapitated. In the control group, unstressed animals were handled daily, and on the day of the experiment, they were sacrificed at the same time as the stressed animals.
The OF box was a 90×90×45 cm wooden box used to study the cognitive and emotional reaction by observing the animal's behaviors.15 Its bottom was divided into 5×5 cm squares; the square in the middle was designated the center square, the others were peripheral squares. For the stress test, eight rats were selected randomly from the two age groups used for the CMRS test. The OF test was performed on day 1, 2, 3, 4, 5, 10, 15, and 21, immediately following the CMRS test. Each animal was placed in the center of the OF box and observed for 3 minutes. The indices of the test included the number of square crossings, number of grooming events, time spent on the center square, vertical movement scores, and number of stools produced. All indices were observed and recorded by two individuals who did not know the purpose of this test. The OF box was cleaned after each test session.
To determine the efficacy of different times of stress application, plasma corticosterone was analyzed. Before the rats were killed, blood samples were collected by intracardiac puncture and were centrifuged at 4℃ to separate the plasma, which was stored at - 20℃ until assayed for corticosterone. Plasma corticosterone levels were measured by a radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA, USA). The intra-assay variability of the RIA ranged between 3.1 and 4.5%. The sensitivity of the assay was 5.7 ng/mL.
Animals of young and aged groups were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and sacrificed immediately by decapitation at each point of 15, 30, 60, 180, and 720 min after stress, and control rats were killed under the same condition (0 min, basic expression). Their brains were removed for isolating the hippocampal tissues. For each rat, 50-100 mg hippocampal tissue was abraded and lysed in 1 mL tissue and cell lysis solution,16 then centrifuged at 15,000 g for 15 min at 4℃. The protein content of the supernatant was determined using the Bradford assay,16 then an equal amount of protein from each sample was subjected to 15% SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane and detected using an Enhanced Chemiluminescent Method (ECM) of Western Blotting system. An immunoblotting reaction was performed with a rabbit anti-BDNF polyclonal antibody (1 : 400 dilution in 1×TBS-T, and the total volume was 4,000 µL) overnight at 4℃; horseradish peroxidase (HRP-labeled goat anti-rabbit IgG) was used as the secondary antibody (1 : 10,000 dilution in 1×TBS-T, and the total volume was 5,000 µL). The expression of BDNF was determined by calculating the density ratio of BDNF to a β-actin band (the software of Image J was used to analyze the density ratio and the software was downloaded from http://rsb.info.nih.gov/ij/download.html). Anti-BDNF antibody, anti-β-actin antibody, and the secondary antibody were purchased from Wuhan Boster Company (http://www.boster.com.cn/).
Hippocampal tissues were obtained at a total of 6 time points from young and aged groups. Total mRNA was extracted from 50-100 mg hippocampus according to the instructions of the TRIzol kit (Invitrogen).The RT-PCR kit was purchased from Promega. The primers for BDNF and TrkB were synthesized by Sbsgene Company, and the sequences of the primers were as follows:
5'AGTGATGACCATCCTTTTCCTTAC3'plus
5'CCTCAAATGTGTCATCCAAGGA3'(196bp, for BDNF)
and
5'GGCCAAGAATGAATATGGTAA 3'plus
5'TTGAGCTGGCTGTTGGTGAT 3'(485bp, for trkB)
and
5'CACAGCTAGAGGGAAATCG3'plus 5'CACCAGAGTAGTTGCGCTC 3'(348bp, for β-actin).
Reactions were performed in a 50 µL volume containing 1 µL AMV reverse transcriptase, 1 µL TflDNA polymerase, 2 µL 25 mM MgSO4, 2 µL primers, 1 µL dNTP Mix, 10 µL 5×Reaction Buffer, and 31 µL Nuclease-Free Water. The reaction sequence was 45℃ for 45 min (1 cycle), 94℃ for 2 min (1 cycle), 94℃ for 30 sec, 55℃ for 1 min, 68℃ for 2 min (30 cycles), 68℃ for 7 min (1 cycle), then a 4℃ soak. PCR products were analyzed by electrophoresis and the density ratio of target genes to the β-actin band was used to determine the levels of expression.
SPSS 11.0 software (SPSS Inc, Chicago, IL, USA) was used for calculations in this study. Mean and SEM were calculated from 6 animals per group for BDNF and TrkB mRNA. The quantitative data were expressed as Mean ± SD and statistical analysis was performed by t-tests between individual groups. One-way ANOVA was used to analyze the different expression of BDNF mRNA between young and aged groups of rats.
Fig. 2 shows that there were no significant differences in exploratory behaviors among handled and control animals in either age group prior to imposition of stress. With prolongation of the experimental days, the quadrant crossing, grooming, and vertical movement scores of young and aged CMRS group animals exhibited a downward trend, and the time spent in the center square clearly increased. The OF indices of the aged CMRS group showed significant decreases compared with the young group, and displayed depression-like behavior and weak stress resilience (Figs. 2 and 3).
There was a large, rapid, and significant increase in plasma corticosterone levels after 15 min of AS, both in the young and the aged groups (294 ± 27 ng/mL vs. 21 ± 7 ng/mL in young control group, t = 42.89, p < 0.001; 197 ± 31 ng/mL vs. 9 ± 2 ng/mL in aged control group, t = 13.36, p < 0.001), and the highest peak of corticosterone present, respectively, at 30 min in young group and 60 min in aged groups after stress (466 ± 43 ng/mL; 328 ± 22 ng/mL), which all maintained high levels until 180 min after stress (Fig. 4). The levels of plasma corticosterone in the two CMRS groups also showed increases after stress, and reached maximal concentrations 30 min after stress (193 ± 38 ng/mL in young group; 104 ± 20 ng/mL in aged group), which were lower than those of AS groups (Fyoung = 26.58, p < 0.01; Faged = 25.46, p < 0.01) (Fig. 5).
Western blotting was performed in the hippocampus to examine discrete modifications of the BDNF protein on control groups (0 min) and stress-exposed groups at 15, 30, 60, 180, and 720 min after stress. After AS, we observed a rapid increase in BDNF at 15 min (as well as 30 and 60 min) throughout the entire hippocampus, in both young and aged groups. A decrease occurred at 180 min and the levels had approached the original condition by 720 min. The maximum expression of BDNF in both young and aged AS groups were observed at 30 min (Fig. 6). After chronic repeated stress, the expression of BDNF showed a dynamic change at 15, 30, 60, 180, and 720 min, whereas the levels were lower than those measured in both the young and aged control groups. However, we observed that the BDNF protein level detected in the young group, 15 min after the last repeated stimulus, was higher than that measured at all other time points, and displayed a remarkable change from 15 min to 180 min. In contrast, the expression level in the aged group was lower than that of the young group at several time points and showed a similarly low level from 60 min to 720 min (Fig. 7).
To evaluate variations in the expression of BDNF and TrkB transcripts after different stresses, these were analyzed at 15, 30, 60, 180, and 720 min after stress by RT-PCR. AS application resulted in rapid up-regulation of BDNF mRNA, with a maximum expression occurring after 15 min of stress in both young and aged groups. There was a significant decrease in expression at 60 min in the aged group, but not in the young group (Fig. 8). No quantitative changes in TrkB mRNA were observed after short periods of AS, although a slight increase was seen at 30 min (Fig. 9). In contrast, following chronic stress, BDNF mRNA measured at five points exhibited decreased expression compared with those of the control groups, but the TrkB mRNA was significantly augmented in the young group during the time course after stress (Figs. 10 and 11). The levels of BDNF and TrkB transcript expression in aged rats were lower than those measured in the initial condition in aged control animals. The levels of BDNF mRNA dec ± reased after chronic stress, whereas TrkB mRNA had increased.
Animal behaviors clearly change in response to stressful conditions.17 Forced-swimming, a relatively mild physiological stress that causes no body damage, was used to build the current animal model. An OF test was used to evaluate rat behavioral changes following exposure to chronic stress. Quadrant crossing is a locomotor activity that shows the rat's movements in an open field, while grooming is an emotional response that indicates the tension of the rats in a new environment. These indices help us to evaluate the behavioral changes of rats in response to stress.18,19
In the current study, two factors led to the behavioral disturbances, namely, aging and the duration of stress. We observed that both young and aged CMRS groups displayed a decrease in square crossing, grooming behavior, and vertical movements, as well as an increase in stool production and increased retention time in the middle square compared with control groups after stress, which represented an inhibition of activities and an increase in emotional disorders. Furthermore, the behavioral activity of aged animals was noticeably weaker than that of young ones in several aspects, representing an obviously abepithymia state. Animals showed little interest in, and weak adaptability to, new surroundings under a chronic stress state. Moreover, behaviors of aged animals were fewer in number than those observed in the young group, reflecting a weaker tolerance to stress. Stress had effect on the function of pre-frontal cortex and limbic system, which were closely bound up with cognition and behavioral response.20 The level of plasma corticosterone in aged animals obviously rose, which acted on the down-regulation of the hippocampal glucocorticoid (GC) receptor synthesis and receptor binding,21,22 and the disorder of negative feedback function of hypothalamo-pituitary-adrenal (HPA) axis.22,23 Thus, senescence led to the enhanced sensitivity of animals to stress, which may result in a serious stress injury and changes of behaviors.
The limbic-hypothalamopituitary-adrenal (LHPA) axis plays an important role in stress and is the ultimate nerve pathway operating during stress responses. Corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and GCs are important hormones in the HPA axis. GCs are the key hormones involved in stress adaptation because of their influence on multiple organs.24 GCs are known to exert feedback regulation on the functioning of the HPA axis, and can increase the release of excitatory amino acids.25 This leads to excitability toxicity of hippocampus, which is characterized by abnormal form of pyramidal neurons, neuronal atrophy, and significant decreases in cell number.26 Therefore, plasma corticosterone expression can be viewed as a symptom of stress injury during exposure to a stressor. Previous studies have indicated that 3 h incubation of hippocampal neurons with GCs depresses activity-dependent expression of BDNF mRNA.27,28 Similarly, Lauterborn, et al. reported that adrenal hormones inhibit in vivo BDNF expression.29 In contrast, Marmigère, et al.30 demonstrated that plasma corticosterone levels were strongly increased after 15, 60, and 180 min of stressor exposure, and were accompanied by a high expression of BDNF.31 In the present report, corticosterone levels in peripheral blood detected rapidly increased in response to AS at 15, 30, and 180 min in both young and aged groups, indicating an intensive stress state. Plasma corticosterone levels determined in the chronic groups also showed increases at 15 and 30 min after stress, but these levels declined to control levels over time, affirming the stress efficacy. Senescence and stress both resulted in the increase of the corticosterone,32,33 which led to the hyperfunction of hippocampus and functional deficit of learning, memory, and cognition. Meanwhile, the hippocampal neuronatrophy that was induced by sustained high concentration of GCs had an effect on the decrease of BDNF mRNA expression.34,35
BDNF is a NTF involved in critical CNS function, as well as synaptic transmission and plasticity, and it plays an important role in the survival, maintenance, and growth of neurons.36-38 The results of the present study demonstrate that AS induced a rapid increase in the expression of BDNF, BDNF mRNA, and TrkB mRNA in the hippocampus, in both young and aged animals. Rapid variations in BDNF protein induced by AS were observed at 15, 30, and 60 min after stress, which was followed by a downturn to the initial condition by 180 min. Overall, the expression of BDNF in the aged group was significantly less than that determined in the young group at 15 and 30 min. Increased BDNF mRNA levels have been previously reported, after 60 min immobilization stress, and decreased levels after 2 h stress, in the dentate gyrus, the CA3 area, and the entire hypothalamus.17,39,40 However, the expression of TrkB mRNA was not influenced by acute immobilization,35 which is contrary to the observations in the present study. In contrast, chronic repeated stress led to a notable increase in TrkB mRNA, but an obvious decrease in BDNF mRNA and proteins in the previous work.17
To fully confirm the specificity of the early increase in BDNF detected in the present report, BNDF mRNA and TrkB mRNA were measured by RT-PCR. The mRNA results confirmed that the augmentation of BDNF mRNA had already occurred 15 min after stress. No substantial increase in TrkB mRNA was seen, although there was a slightly enhanced expression compared with basic condition.
BDNF and its receptor TrkB play important roles during stress injury. After a series of acute injuries, such as cerebral ischemia, epilepsy, or cerebral trauma, the upregulation of BDNF mRNA, as well as the augmentation of TrkB mRNA, were observed in the cerebral cortex and hippocampus.31,41 Similarly, Fujhara, et al.42 demonstrated that the levels of BDNF protein and mRNA increased significantly in the hippocampus of rats that had been treated with short-term sleep-disturbance.43 Moreover, increased BDNF mRNA and protein levels occurred in the pituitary glands of rats stressed for 60 min, while decreased levels occurred following stress for 180 or 300 min.42 Scaccianoce, et al. and Smith, et al. demonstrated by RT-PCR and in situ hybridization that AS induced down-regulation of BDNF mRNA.35,44 Most researchers now view long-term and chronically repeated stress as making significant contributions to decreased expression of BDNF, as well as increased expression of TrkB mRNA. The decreased expressions of BDNF mRNA and protein were observed in the CA3 and the dentate gyrus of the hippocampus after repeated immobilization stress,45,46 together with increased expression of TrkB mRNA.
In the present study, the expression of BDNF protein and mRNA showed a significant decrease at 60, 180, and 720 min after CMRS. The levels of BNDF mRNA detected in both young and aged CMRS groups were lower than those of acute groups at several time points after stress. The expression levels of BDNF and TrkB determined in the aged groups were lower than those measured in the young group at several time points, as analyzed by Western blotting and RT-PCR. Thus, although not definitive, the results suggested that BDNF and its receptor were influenced by both the stress paradigm and by senescence. The up-regulation of TrkB mRNA measured in the CMRS groups may be a compensatory adaptation to repeated stress, similar to the change of BDNF detected in the AS groups.
We now know that unexpected and AS tends to provide an excited organism with a degree of protection for a short time. This suggests that the augmented expression of BDNF mRNA and protein might, either directly or indirectly, contribute to stress protection. However, with prolongation of the stress, if the excitation is not attenuated, secondary neuronal damage would occur.47 Thus, a second response that leads to a decrease in BDNF mRNA and protein expression occurs. The observed up-regulation of TrkB mRNA might be a further compensatory adaptation to prolonged stress-induced down-regulation of BDNF. Up-regulation of TrkB could possibly make the neurons of the hippocampus more responsive to lower levels of BNDF than are induced by chronic stress.44,46 The present study showed that the expression of BDNF and TrkB were associated with aging, which is known to attenuate mammalian stress coping capacity.48-50 The expression of BDNF and TrkB has been reported to increase during learning-related events and to decrease in the hippocampus of alzheimer's disease (AD) patients, suggesting that these are involved in learning, emotion, and age-related memory deficits. Although Lapchak, et al. determined that age did not change the prevalence or regional distribution patterns of BDNF or TrkB mRNA in the hippocampal formation throughout the lifespan of male rats,51-53 more research has shown that significant decreases with age can be detected for BDNF mRNA and TrkB mRNA in many areas of the brain.2,51,54
We compared the dynamic change of BDNF and TrkB expression in the hippocampus of young and aged rats after different stress applications in order to illustrate the effect of senescence on neurotrophin expression. A significant increase in BDNF mRNA and protein occurred both in young and aged rats during the time course after AS, accompanied by a slight change in TrkB. The levels of the aged group were generally lower than were those of the young group. Similarly, the dynamic change of BDNF and TrkB expression that was detected in chronically stressed groups was slighter than that of AS groups, and there was a distinct reduction in response in the aged group compared to the young group. Hock and Hosinger suggested that the induction of BDNF and TrkB mRNA detected in the hippocampus of AD was substantially lower than that seen in normal adult cellular tissues.55,56 More importantly, the spread of BDNF in the AD hippocampus was similar to that in naturally aging animals, suggesting that a low content of BDNF in the brain was one of the reasons for cognitive disorders and weak responsiveness to stress. Thus, we also concluded that aging could reduce the expression level of BDNF and TrkB mRNA, which might contribute to poor stress protection seen in the present study in aged rats.57
In conclusion, alterations of BDNF and its receptor of TrkB were influenced by several factors, such as a stress paradigm, stress duration, and aging. The rapid increase in BDNF mRNA, protein, and TrkB mRNA in response to AS may be part of a neuronal protective response. The decreased expression measured in aged animals exposed to chronic stress may indicate a natural loss of this protective function during senescence. Clearly, further study will be necessary to confirm the expression of BDNF and its high affinity receptors and to explore the mechanisms in up/down-regulation of BDNF expression in different brain areas, in order to further understand the complexities of neuronal responses to stress.
Figures and Tables
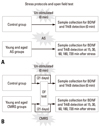 | Fig. 1Diagrammatic representation of the protocols for AS and CMRS exposures. (A) The AS protocol. (B) The CMRS protocol. AS, acute stress; CMRS, chronic mild repeated stress. |
 | Fig. 2The results of the open field test for young CMRS group rats (Mean ± SD) (n = 6). *p < 0.05, **p < 0.001, different from control groups (Student's t-test). (A) Number of square crossings in different stress periods. (B) Vertical movements in different stress periods. (C) Number of grooming events in different stress periods. (D) Time spent in the center square during a stress course. CMRS, chronic mild repeated stress. |
 | Fig. 3The results of an open field test for aged CMRS group rats (Mean ± SD) (n = 6). *p < 0.05, **p < 0.001, †p < 0.05, ‡p < 0.001, different from control groups (Student's t-test). (A) Number of square crossings in different stress periods. (B) Vertical movements in different stress periods. (C) Number of grooming events in different stress periods. (D) Time spent in the center square during a stress course. CMRS, chronic mild repeated stress. |
 | Fig. 4Temporal profile of plasma corticosterone (ng/mL) levels of young and aged AS group rats. Values are shown for control animals (0 min, n = 6) and after 15, 30, 60, 180 and 720 min of stress. The results are expressed as mean ± SEM. *p < 0.001 vs. young control group. **p < 0.001 vs. aged control group. AS, acute stress. |
 | Fig. 5Temporal profile of plasma corticosterone (ng/mL) levels in young and aged CMRS rat groups. Values are shown for control animals (0 min) and after 15, 30, 60, 180 and 720 min of stress. The results are expressed as mean ± SEM. *p < 0.001 vs. young control group. **p < 0.01 and †p < 0.001 vs. aged control group. CMRS, chronic mild repeated stress. |
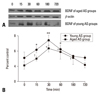 | Fig. 6Time course of BDNF protein expression in the hippocampus measured by Western blotting after acute stress (AS). (A) Representative photographic film, illustrating the dynamic changes in BDNF protein from young and aged AS groups. (B) Results of statistical analysis of BDNF protein expression in control groups (unstressed, 0 min) and stressed animals after 15, 30, 60, 180, 720 min of stress (following acute or chronic stress imposition). The results were expressed as mean ± SEM. *p < 0.05 and **p < 0.001 vs. young control group. †p < 0.05 and ‡p < 0.001 vs. aged control group. n = 5-6 rats per each time point studied in two independent stress conditions. BDNF, brain-derived neurotrophic factor; AS, acute stress. |
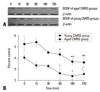 | Fig. 7Time course of BDNF protein expression in the hippocampus measured by Western blotting after chronic repeated stress. (A) Representative photographic film, illustrating the dynamic changes in BDNF protein from the young and aged CMRS groups. (B) Results of statistical analysis of BDNF protein expression in control groups (unstressed, 0 min) and stressed animals after 15, 30, 60, 180, 720 min of stress (in two stress conditions). The results were expressed as mean ± SEM. *p < 0.05 and **p < 0.001 vs. young control group. †p < 0.05 and ‡p < 0.001 vs. aged control group. n = 5-6 rats per each time point studied in two independent stress conditions. BDNF, brain-derived neurotrophic factor; CMRS, chronic mild repeated stress. |
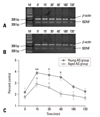 | Fig. 8BDNF mRNA expression detected by semiquantitative RT-PCR in control groups (unstressed, 0 min) and young and aged AS groups after a period of different stress performance. Total RNA was isolated from hippocampus and assayed for BDNF at 15, 30, 60, 180, 720 min after stress. (A) Representative electrophoretograms illustrating the expression of BDNF mRNA in the young control and AS groups. (B) Expression of BDNF mRNA in the aged AS group. (C) Quantitative analysis of BDNF mRNA at different time points after stress. The results were calculated as the intensity of the lane of each transcript over the intensity of the β-actin (internal standard) band and expressed as the mean ± SEM. *p < 0.05 and **p < 0.001 vs. young control group. †p < 0.05 and ‡p < 0.001 vs. aged control group. n = 5-6 rats per each time point studied in two independent stress conditions. BDNF, brain-derived neurotrophic factor; AS, acute stress; RT-PCR, reverse transcription-polymerase chain reaction. |
 | Fig. 9TrkB mRNA expression measured by semiquantitative RT-PCR in the control groups (unstressed, 0 min) and young and aged AS groups after a period of different stress performance. Total RNA was isolated from the hippocampus and assayed for TrkB mRNA at 15, 30, 60, 180, 720 min after stress. (A) Representative electrophoretograms showing the expression of TrkB mRNA in the young control (0 min) and AS groups. (B) TrkB mRNA expression in the aged control and AS groups. The results were calculated as the intensity of the lane of each transcript over the intensity of β-actin (internal standard) band and expressed as the mean ± SEM. n = 5-6 rats per each time point studied. TrkB, tyrosine kinase-coupled receptor; RT-PCR, reverse transcription-polymerase chain reaction; AS, acute stress. |
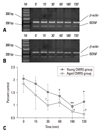 | Fig. 10BDNF mRNA expression detected by RT-PCR in the control groups (unstressed, 0 min) and young and aged CMRS groups after a period of different stress performance. Total RNA was isolated from the hippocampus and assayed for BDNF at 15, 30, 60, 180, 720 min after stress. (A) Representative electrophoretograms showing the expression of BDNF mRNA in the young control and CMRS groups. (B) BDNF mRNA expression in the aged control and CMRS groups. (C) Quantitative analysis of BDNF mRNA at different time points after stress. The results were calculated as the intensity of the lane of each transcript over the intensity of β-actin (internal standard) band and expressed as the mean ± SEM. *p < 0.05 and **p < 0.001 vs. young control group. †p < 0.05 and ‡p < 0.001 vs. aged control group. n = 5-6 rats per each time point studied in two independent stress conditions. BDNF, brain-derived neurotrophic factor; CMRS, chronic mild repeated stress; RT-PCR, reverse transcription-polymerase chain reaction. |
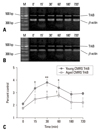 | Fig. 11TrkB mRNA expression measured by semiquantitative RT-PCR in the control groups (unstressed, 0 min) and young and aged CMRS groups after a period of different stress performance. Total RNA was isolated from the hippocampus and assayed for TrkB at 15, 30, 60, 180, 720 min after stress. (A) Representative electrophoretograms showing the expression of TrkB mRNA in the young control and CMRS groups. (B) TrkB mRNA expression in the aged control and CMRS groups. (C) Line chart represented the results of quantitative analysis of TrkB mRNA at different time points after stress. The results were calculated as the intensity of the lane of each transcript over the intensity of β-actin (internal standard) band and expressed as the mean ± SEM. *p < 0.05 and **p < 0.001 vs. young control group. †p < 0.001 vs. aged control group. n = 5-6 rats per each time point studied in two independent stress conditions. TrkB, tyrosine kinase-coupled receptor; CMRS, chronic mild repeated stress; RT-PCR, reverse transcription-polymerase chain reaction. |
ACKNOWLEDGEMENTS
This work was supported by the Technological Study Project of Shandong Province (2007GG20002018).
References
1. Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989. 341:149–152.

2. Croll SD, Iq NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998. 812:200–208.

3. Connor B, Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Rev. 1998. 27:1–39.

4. Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextural learning. Nat Neurosci. 2000. 3:533–535.

5. Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003. 27:893–903.
7. Aggleton JP, Vann SD, Oswald CJ, Good M. Identifying cortical inputs to the rat hippocampus that subserve allocentric spatial processes: a simple problem with a complex answer. Hippocampus. 2000. 10:466–474.

8. McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006. 8:367–381.

9. Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004. 124:985–992.

10. Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol. 2009. 12:73–82.

11. Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997. 28:103–110.

12. Lee T, Saruta J, Sasaquri K, Sato S, Tsukinoki K. Allowing animals to bite reverses the effects of immobilization stress on hippocampal neurotrophin expression. Brain Res. 2008. 1195:43–49.
13. Gray JA. The psychology of fear and stress. 1987. New York: Cambridge University Press.
14. de Cabo de la Vega C, Pujol A, Paz Viveros M. Neonatally administered naltrexone affects several behavioral responses in adult rats of both genders. Pharmacol Biochem Behav. 1995. 50:277–286.

15. Sarkisova KYu, Kulikov MA. Prophylactic actions of the antioxidant agent AEKOL on behavioral (psychoemotional) disturbances induced by chronic stress in rats. Neurosci Behav Physiol. 2001. 31:503–508.
16. Srisomsap C, Sawangareetrakul P, Subhasitanont P, Panichakul T, Keeratichamroen S, Lirdprapamongkol K, et al. Proteomic analysis of cholangiocarcinoma cell line. Proteomics. 2004. 4:1135–1144.

17. Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999. 830:56–71.
18. Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008. 107:522–532.
19. Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl). 2001. 158:100–106.

20. Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005. 156:105–114.

21. Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, et al. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999. 19:2356–2361.

22. McQuade R, Young AH. Future therapeutic targets in mood disorders: the glucocorticoid receptor. Br J Psychiatry. 2000. 177:390–395.

23. Hassan AH, Patchev VK, von Rosenstiel P, Holsboer F, Almeida OF. Plasticity of hippocampal corticosteroid receptors during aging in the rat. FASEB J. 1999. 13:115–122.

24. Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001. 25:117–142.

25. Powers SK, LOCKE And M, Demirel HA. Exercise, heat shock proteins, and myocardial protection from I-R injury. Med Sci Sports Exerc. 2001. 33:386–392.
26. Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000. 97:253–266.
27. Cosi C, Spoerri PE, Comelli MC, Guidolin D, Skaper SD. Glucocorticoids depress activity-dependent expression of BDNF mRNA in hippocampal neurons. Neuroreport. 1993. 4:527–530.
28. Makino S, Kaneda T, Nishiyama M, Asaba K, Hashimoto K. Lack of decrease in hypothalamic and hippocampal glucocorticoid receptor mRNA during starvation. Neuroendocrinology. 2001. 74:120–128.

29. Lauterborn J, Berschauer R, Gall C. Cell-specific modulation of basal and seizure-induced neurotrophin expression by adrenalectomy. Neuroscience. 1995. 68:363–378.

30. Marmigére F, Givalois L, Rage F, Arancibia S, Tapia-Arancibial L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003. 13:646–655.

31. Rage F, Givalois L, Marmigére F, Tapia-Arancibia L, Arancibia S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience. 2002. 112:309–318.

32. Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998. 1:69–73.

33. Hibberd C, Yau JL, Seckl JR. Glucocorticoids and the ageing hippocampus. J Anat. 2000. 197:553–562.

35. Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995. 15:1768–1777.

37. Yamasaki Y, Shigeno T, Furukawa Y, Furukawa S. Reduction in brain-derived neurotrophic factor protein level in the hippocampal CA1 dendritic field precedes the delayed neuronal damage in the rat brain. J Neurosci Res. 1998. 53:318–329.

38. Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002. 70:735–744.

39. McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999. 22:295–318.

40. Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999. 267:81–84.

41. Tsukinoki K, Saruta J, Sasaguri K, Miyoshi Y, Jinbu Y, Kusama M, et al. Immobilization stress induces BDNF in rat submandibular glands. J Dent Res. 2006. 85:844–848.

42. Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003. 21:223–232.
43. Grundy PL, Patel N, Harbuz MS, Lightman SL, Sharples PM. Glucocorticoids modulate BDNF mRNA expression in the rat hippocampus after traumatic brain injury. Neuroreport. 2000. 11:3381–3384.

44. Scaccianoce S, Del Bianco P, Caricasole A, Nicoletti F, Catalani A. Relationship between learning, stress and hippocampal brain-derived neurotrophic factor. Neuroscience. 2003. 121:825–828.

45. Givalois L, Marmigère F, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. Immobilization stress rapidly and differentially modulates BDNF and TrkB mRNA expression in the pituitary gland of adult male rats. Neuroendocrinology. 2001. 74:148–159.

46. Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999. 267:81–84.

47. Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005. 53:129–139.
48. Gutsmann-Conrad A, Pahlavani MA, Heydari AR, Richardson A. Expression of heat shock protein 70 decreases with age in hepatocytes and splenocytes from female rats. Mech Ageing Dev. 1999. 107:255–270.

49. Honma Y, Tani M, Takayama M, Yamamura K, Hasegawa H. Aging abolishes the cardioprotective effect of combination heat shock and hypoxic preconditioning in reperfused rat hearts. Basic Res Cardiol. 2002. 97:489–495.
50. Alsbury S, Papageorgiou K, Latchman DS. Heat shock proteins can protect aged human and rodent cells from different stressful stimuli. Mech Ageing Dev. 2004. 125:201–209.

51. Lapchak PA, Araujo DM, Beck KD, Finch CE, Johnson SA, Hefti F. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol Aging. 1993. 14:121–126.
52. Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Down-regulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998. 813:112–120.

53. Narisawa-Saito M, Nawa H. Differential regulation of hippocampal neurotrophins during aging in rats. J Neurochem. 1996. 67:1124–1131.
54. Sihol M, Arancibia S, Perrin D, Maurice T, Alliot J, Tapia-Arancibia L. Effect of aging on brain-derived neurotrophic factor, proBDNF, and their receptors in the hippocampus of Lou/C rats. Rejuvenation Res. 2008. 11:1031–1040.
55. Hosinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000. 76:347–354.

56. Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000. 57:846–851.

57. Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005. 132:613–624.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download