Abstract
Purpose
The ketogenic diet has long been used to treat epilepsy, but its mechanism is not yet clearly understood. To explore the potential mechanism, we analyzed the changes in gene expression induced by the ketogenic diet in the rat kainic acid (KA) epilepsy model.
Materials and Methods
KA-administered rats were fed the ketogenic diet or a normal diet for 4 weeks, and microarray analysis was performed with their brain tissues. The effects of the ketogenic diet on cathepsin E messenger ribonucleic acid (mRNA) expression were analyzed in KA-administered and normal saline-administered groups with semi-quantitative and real-time reverse transcription polymerase chain reaction (RT-PCR). Brain tissues were dissected into 8 regions to compare differential effects of the ketogenic diet on cathepsin E mRNA expression. Immunohistochemistry with an anti-cathepsin E antibody was performed on slides of hippocampus obtained from whole brain paraffin blocks.
Results
The microarray data and subsequent RT-PCR experiments showed that KA increased the mRNA expression of cathepsin E, known to be related to neuronal cell death, in most brain areas except the brain stem, and these increases of cathepsin E mRNA expression were suppressed by the ketogenic diet. The expression of cathepsin E mRNA in the control group, however, was not significantly affected by the ketogenic diet. The change in cathepsin E mRNA expression was greatest in the hippocampus. The protein level of cathepsin E in the hippocampus of KA-administered rat was elevated in immunohistochemistry and the ketogenic diet suppressed this increase.
The ketogenic diet has been in clinical use for over 80 years for the symptomatic treatment of epilepsy.1 Although its clinical use declined after the introduction of antiepileptic drugs, the ketogenic diet has undergone a resurgence and has gained popularity since it was discovered that it is an effective therapy for intractable epilepsy, especially in pediatric patients.2 Many clinical studies have shown its efficacy,3 and the ketogenic diet is now widely used around the world.4 The ketogenic diet is a high fat, low-carbohydrate diet that induces ketoacidosis, mimicking the metabolic state of starvation. Although its clinical relevance is well documented, its mechanism remains obscure.5 To investigate potential molecular mechanisms, we analyzed changes in gene expression induced by the ketogenic diet in a kainic acid (KA) animal model. KA, an analogue of glutamate, is an excitotoxin that can induce epilepsy in animals similar to human temporal lobe epilepsy.6,7 Administration of KA in the rodent results in neuronal cell death mainly in the limbic system.8 The ketogenic diet is known to be effective in KA-induced epilepsy9 and to protect neuronal cell death in the hippocampus of KA-administered mice.10 Here, we performed a DNA microarray analysis to elucidate the effect of the ketogenic diet on gene expression in the KA animal model. In the microarray analysis, several genes including cathepsin E were down-regulated by the ketogenic diet in KA-administered rats. Cathepsin E is an intracellular, nonlysosomal aspartic proteinase that has been associated with brain ischemia,11 brain aging,12 neuronal degeneration,13 and excitotoxin-induced neuronal cell death.14 When KA was injected into the ventricle of rat brain, a marked elevation in the messenger ribonucleic acid (mRNA) level of cathepsin E, followed by increases of both matured and modified cathepsin E proteins was observed in the hippocampus,14 which is known to be an important brain region in epileptogenesis. Hence, we investigated the correlation between cathepsin E expression and the ketogenic diet in the KA animal model.
The ketogenic diet consisted of 78.85% (w/w) lipid, 9.5% (w/w) protein, 0.76% (w/w) carbohydrate, 5% (w/w) cellulose, 3.8% (w/w) mineral mix (AIN-76), and 2.09% (w/w) vitamin mix (AIN-76A)15 and was purchased from Dyets Inc. (Bethlehem, PA, USA). At the beginning of an experiment, animals were fasted for 24 hours and then fed the ketogenic diet (ad libitum) or a normal diet (ad libitum).
Male Sprague-Dawley rats were obtained from SLC Inc. (Shizuoka, Japan), housed 3 or 4 to a cage, kept in a temperature-controlled room at 22℃ with 12-hour day-night cycles (lights on at 7 a.m.) , and weighed every week. All rats were 5 weeks old on arrival and 6 weeks old at the beginning of the experiment. KA (8 mg/kg) was administered i.p. to rats of the experimental group (n = 20), and saline was administered to rats of the control group (n = 10).16 The half rats of the experimental and control groups were fed the ketogenic diet starting 2 days after the injection, and the other half of each group were fed a normal diet. The diet continued to be provided for 4 weeks, after which the animals were sacrificed and the brains were removed intact or dissected into 8 regions: frontal cortex, posterior cortex, striatum, hippocampus, thalamus, brain stem, cerebellum, and hypothalamus, amygdala, septum, and preoptic area (HASP) to analyze differential expression of genes in different brain areas.17 Whole brains (n = 3 for each group) or brain regions (n = 3 for each group) were mixed and homogenized for the microarray experiment or reverse transcription polymerase chain reaction (RT-PCR). For immunohistochemistry experiment, KA was administered i.p. to rats with different doses of 12 mg/kg (n = 6) or 25 mg/kg (n = 6), and normal saline to control rats (n = 6). Four out of 6 rats were dead in the higher-dose group (25 mg/kg) after KA injection, whereas no rat was dead in the lower-dose group (12 mg/kg). The half of each group was fed a normal diet or the ketogenic diet for 4 weeks as described above.
Blood samples were obtained from rat tails every week. Blood levels of β-hydroxybutyrate (β-HBA) were assayed with a β-HBA kit (Sigma Diagnostics, St Louis, MO, USA) according to the manufacturer's protocol. Briefly, 5 µL of serum was mixed with 0.3 mL of β-HBA reagent, a component of the kit, and the initial absorbance (OD345) was immediately measured. After the samples were incubated at 37℃ for 15 minutes with 0.25 U β-HBA dehydrogenase, their absorbance (OD345) was obtained again. The concentration of β-HBA for each sample was calculated from the difference of these two absorbances by comparison with standard solutions.
The effects of a normal diet and the ketogenic diet on gene expression in the brains of KA-injected rats were compared using the microarray technique. Total ribonucleic acid (RNA)(40 µg) was isolated from homogenized brain tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and sent to Digital Genomics Inc. (Seoul, Korea) for microarray analysis. The TwinChip™ Rat 5K (Digital Genomics), which has duplicated spots for 4,283 rat genes on each chip, was used for the microarray analysis. The probe was labeled with Cy3 for the normal-diet rats and with Cy5 for the ketogenic-diet rat. The means of the data from each pair of duplicated spots were used for comparing gene expression. All microarray data discussed in this publication have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE 19277 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19277).
Total RNAs (1 µg) isolated from each sample were used for complementary deoxyribonucleic acid (cDNA) synthesis with SuperScript II reverse transcriptase (Invitrogen). The following primers were used to measure the mRNA level of genes. In the semiquantitative RT-PCR analysis, for cathepsin E: 5'-ACTGTGATGTTCTGCTCTGAGGGCTG-3' (sense) and 5'-GCTGCGTGGCTATTTATCACACCATT-3' (antisense), for β-actin: 5'-CCCAGAGCAAGAGAGGCATCCT-3' (sense) and 5'-ACGCACGATTTCCCTCTCAGCT-3' (antisense). In the real-time RT-PCR analysis, for cathepsin E: 5'-TCAATGAGCCCCTCATCAACTACC-3' (sense) and 5'-TGGATGAGCCCGTGTCAAAGAT-3' (antisense), for β-actin: 5'-ACCACACTTTCTACAATGAGCTGCG-3' (sense) and 5'-TGGGTCATCTTTTCACGGTTGG-3' (antisense). Semiquantitative PCR amplification was performed for 25 cycles (94℃ for 30 seconds, 63℃ for 30 seconds, and 72℃ for 30 seconds for cathepsin E; 94℃ for 30 seconds, 55℃ for 30 seconds, and 72℃ for 30 seconds for β-actin). Real-time PCR reactions were performed in triplicate and subjected to 45 cycles (94℃ for 15 seconds, 55℃ for 30 seconds, and 72℃ for 30 seconds) in the presence of 1×SYBR Green mix (Applied Biosystems, Foster City, CA, USA) using a Rotor-Gene™ 3,000 (Corbett Life Science, Mortlake, Sydney, Australia). The amount of product was determined at the end of each cycle by the RotorGene software (Corbett Life Science). The relative amounts of cathepsin E mRNA were normalized and calculated using the Pfaffl method.18
Rats were anesthetized with ether and transcardially perfused with neutral buffered formalin (NBF). Whole brains were removed and cut into small pieces, washed overnight, dehydrated, and embedded in paraffin. Embedded brain tissues were sectioned using a microtome, mounted on glass slides, and dried. The avidin-biotin complex (ABC) kit (Vector laboatories, Burlingame, CA, USA) was used to detect the cathepsin E antigen in the brain tissue as described below. After the removal of the paraffin, the tissue sections were incubated with proteinase K for 20 minutes at 37℃ for the purpose of enhancing the reactivity of the primary antibody followed by quenching endogenous peroxidase activity with 3% hydrogen peroxide. After incubation with normal rabbit serum for 1 hour at room temperature, primary antibody reaction was performed for 36 hours in 4℃ with the antibody against cathepsin E (Waco Pure Chemical Industries Ltd., Osaka, Japan) diluted 1 : 100 with phosphat buffer solution (PBS) containing 1% normal rabbit serum. Subsequent incubation with biotinylated secondary antibody and the ABC reagent (Vector laboatories) was performed for 1 hour each at room temperature. For peroxidase substrate reaction, the sections were treated with PBS containing 0.05% diaminobenzidine (DAB) and 0.0015% hydrogen peroxide. The tissue sections were counterstained with the hematoxylin solution if it was necessary. The hippocampal areas were observed and photographed using a BX-50 microscope and DP70 microphotography (Olympus, Tokyo, Japan).
Rats were administered KA (8 mg/kg) or normal saline and fed a normal diet or the ketogenic diet for 4 weeks. The groups were designated normal saline, normal diet (NS-ND), normal saline, ketogenic diet (NS-KD), KA, normal diet (KA-ND), and KA, ketogenic diet (KA-KD). Regardless of KA injection, rats fed the ketogenic diet gained less body weight than rats fed a normal diet (Fig. 1A), which is consistent with previous reports.19,20 The ketogenic diet induced ketoacidosis in the NS-KD and KA-KD groups (Fig. 1B): the concentrations of β-HBA increased from basal levels (< 0.1 mM) to 0.69 ± 0.13 mM (NS-KD rats) and to 1.59 ± 0.17 mM (KA-KD rats) in the first week, whereas the normal diet did not affect the β-HBA concentration (0.03 ± 0.01 mM, NS-ND rats; 0.08 ± 0.02 mM, KA-ND rats). The β-HBA concentration exceeded 2 mM in the second week and increased further over time in KA-KD rats, whereas it remained around 1 mM in NS-KD rats, which is the concentration usually achieved by the ketogenic diet in the normal rats.21
To analyze the changes in gene expression induced by the ketogenic diet in KA-treated rats, total RNA was isolated from the whole brains of KA-ND and KA-KD rats and subjected to a microarray analysis. The mRNA expressions of kinesin family member 5B (Kif5b), cathepsin E, beta1 subunit of adenosine monophosphate (AMP)-activated protein kinase (Prkab1), apolipoprotein B editing protein (Apobec1), and general transcription factor IIa 2 (Gtf2a2) were more than 2 times lower in the ketogenic-diet group than in the normal-diet group (Table 1). In contrast, we did not observe an increase higher than 2-fold in the expression of any gene in the KA-KD group compared to the KA-ND group. Of the down-regulated genes, only cathepsin E is known to be related to epilepsy and this correlation between cathepsin E and KA prompted us to confirm the microarray data further with an RT-PCR analysis. In accordance with the results of the microarray analysis, cathepsin E mRNA expression was lower in the KA-KD rats than in the KA-ND rats in a semiquantitative RT-PCR analysis (Fig. 2).
We compared the effects of the ketogenic diet between groups administered KA and normal saline to determine whether the ketogenic diet also affects cathepsin E expression in the normal rat brain. We also analyzed its differential effect on local brain regions. When the rats were not administered KA, the ketogenic diet did not significantly change cathepsin E mRNA expression (Fig. 3). Although its expressions in the frontal cortex, striatum, and thalamus of NS-KD rats were changed by the ketogenic diet in semi-quantitive RT-PCR analysis, there was no statistically significant difference between NS-ND and NS-KD rats in those areas in real-time RT-PCR. Cathepsin E mRNA expression, however, was significantly increased in most brain areas (p < 0.05), except for the brain stem, when KA was administered to rats fed a normal diet, and all of these increases in cathepsin E mRNA expression were significantly suppressed by the ketogenic diet (p < 0.05) in KA-KD rats. These results suggest that the ketogenic diet affects cathepsin E mRNA expression only when it is abnormally increased by KA. The hippocampus showed the greatest increase in KA-induced cathepsin E mRNA expression (a 5.8-fold increase), and the ketogenic diet decreased its expression in KA-KD rats to 50% of the level of KA-ND rats. However, the cathepsin E mRNA expression in the hippocampus of KA-KD rats still remained high compared to NS-ND or NS-KD rats, although there was no significant difference between KA-KD and NS-ND rats (p = 0.054) or NS-KD rat (p = 0.15). In contrast, the level of cathepsin E mRNA expression in the thalamus of KA-KD rats was significantly higher than that of NS-ND (p = 0.007) or NS-KD rats (p = 0.039).
We performed immunohistochemical staining to confirm the difference of the cathepsin E expression in the neurons of the rat hippocampal region. KA was administered i.p. to rats with two different doses (12 mg/kg or 25 mg/kg) to evaluate the dose-dependent effect of KA on cathepsin E expression. In normal saline-injected rats (NS-ND and NS-KD), neurons positive to cathepsin E protein were barely detected in the cornu ammonis (CA) 3 region of the hippocampus (Fig. 4A and B). In the lower-dose (12 mg/kg) KA-ND rat, a small number of neurons in the stratum oriens of the CA3 region expressed cathepsin E (Fig. 4C). The cathepsin E immunoreactive neurons were also observed in the same region of the lower-dose KA-KD rat in a more or less sparse manner (Fig. 4D). In the higher-dose (25 mg/kg) KA-ND rat, a great number of cathepsin E immunoreactive neurons were observed in the stratum oriens of the CA3 region as well as the CA1 and the CA2 regions (Fig. 4E). With higher magnifications, most of these neurons appeared to be pyramidal neurons, the major output neurons of the hippocampus, although the widespread strong immunoreactivity against cathepsin E throughout the perikaryon of these neurons obscured the detailed morphologies of the nuclei. The increases in the intercellular spaces and the pyknotic changes of neurons were observed (Fig. 4G). In the CA3 region of the higher-dose KA-KD rat, however, the number of cathepsin E-immunoreactive neurons was dramatically decreased by the ketogenic diet and few of them showed immunoreactivity against cathepsin E as strongly as those observed in the higher-dose KA-ND rat (Fig. 4F). These neurons had large, round, euchromatic nuclei, the characteristics of an intact pyramidal neuron (Fig. 4H).
Cathepsin E, an aspartic proteinase, is mainly present in cells of the immune system such as macrophages, lymphocytes, microglia, and dendritic cells. Its physiological role is not yet clearly understood, although it has been proposed to be involved in the immune response because cathepsin E-deficient mice show increased susceptibility to bacterial infections.22 Abnormally elevated levels of cathepsin E have been observed in several tumor types, such as pancreatic ductal adenocarcinoma, cervical adenocarcinoma, gastric cancer, and lung cancer.22 Increased cathepsin E expression has also been observed in aged brains,12 senile plaques of Alzheimer's disease,23 and KA-injected rat brains.14 Our results show that cathepsin E expression increased in the rat brain when KA was administered, and this KA-induced cathepsin E expression was suppressed by the ketogenic diet. To our knowledge, this is the first report that suggests a direct correlation between the ketogenic diet and cathepsin E.
The ketogenic diet is now widely used for the treatment of intractable epilepsy. There is increasing evidence that the ketogenic diet can provide symptomatic and disease-modifying activity in a broad range of neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease.24 It is postulated that ketone bodies confer protection on neurons against diverse types of cell injury.24 Moreover, neuroprotection is now considered to be an important mechanism for the disease-modifying activity of antiepileptic drugs.25,26 Apoptosis is closely related to the neuronal cell death induced by excitotoxins, and proapoptotic events such as activation of bcl-2 family members and DNA laddering occur in status epilepticus-induced neuronal cell death.27 The suppression of apoptosis has been suggested as a mechanism for the neuroprotective effect of the ketogenic diet.28,29 In addition, cathepsin E is known to be involved in the apoptosis of erythrocytes30 and prostate carcinoma cells.31 Moreover, KA induced the widespread pyknosis of CA3 neurons in the hippocampus of KA-ND rats and pyramidal neurons were mostly preserved by the ketogenic diet in the hippocampus of KA-KD rats. It seems reasonable to assume that the suppressive effect of the ketogenic diet on cathepsin E expression may contribute its protective role of the neuron by inhibiting apoptosis. However, no direct evidence of the involvement of cathepsin E in neuronal apoptosis has been yet observed.
It is hard to speculate how the ketogenic diet regulates the transcription of cathepsin E, because little is known about the signaling pathway involved in the increase of cathepsin E expression in the KA-animal model. However, our data imply that the ketogenic diet may suppress cathepsin E expression indirectly, because only KA-induced cathepsin E was suppressed by the ketogenic diet but its basal expression in control rats (NS-ND and NS-KD) was not changed by the ketogenic diet. Recently, several genes were shown to have a pattern of gene expression similar to that of cathepsin E. Clusterin is a proapoptotic protein that accumulates in the rat brain following treatment with KA, and this KA-induced clusterin expression is suppressed by the ketogenic diet.32 Proenkephalin, which contributes to the development of seizures, is induced by KA and down-regulated by the ketogenic diet through the suppression of the JNK signaling pathway.33 These findings including our data suggest that the transcriptional regulation of many molecules induced by excitotoxin may be important for the neuroprotective effect of the ketogenic diet in KA-animal model.
In conclusion, our results showed that cathepsin E expressions induced by KA were suppressed by the ketogenic diet in the rat brain, suggesting that the suppressive effect of the ketogenic diet on cathepsin E expression might contribute to the neuroprotective role of the ketogenic diet in KA-animal model.
Figures and Tables
 | Fig. 1Changes of body weight and serum β-hydroxybutyrate. (A) Groups fed the ketogenic diet (NS-KD and KA-KD) gained less body weight than the control groups fed a normal diet (NS-ND and KA-ND). (B) The serum level of β-hydroxybutyrate increased due to the ketogenic diet in NS-KD and KA-KD rats. NS-ND, normal saline, normal diet; NS-KD, normal saline, ketogenic diet; KA-ND, kainic acid, normal diet; KA-KD, kainic acid, ketogenic diet. |
 | Fig. 2Semiquantitative RT-PCR analysis of cathepsin E mRNA expression in whole brain tissue. Cathepsin E expression was lower in the KA-KD group than in the KA-ND group. RT-PCR, reverse transcription polymerase chain reaction; mRNA, messenger ribonucleic acid; KA-KD, kainic acid, ketogenic diet; KA-ND, kainic acid, normal diet. |
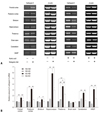 | Fig. 3Cathepsin E mRNA expression in different brain areas. Rat brains were dissected into 8 compartments. (A) In the semiquantitative RT-PCR analysis, the ketogenic diet down-regulated cathepsin E expression in the rats given KA, but not in those given normal saline. (B) In the real-time RT-PCR, KA increased cathepsin E expression in all brain areas examined except the brain stem, and the ketogenic diet suppressed the KA-induced cathepsin E expression. The hippocampus showed the most prominent change in cathepsin E expression. Data represent the relative amount of cathepsin E mRNA of each group compared to NS-ND in the same brain area. *indicates statistical significance (*p < 0.05, **p < 0.01). HASP, hypothalamus, amygdala, septum, and preoptic area. mRNA, messenger ribonucleic acid; RT-PCR, reverse transcription polymerase chain reaction; NS-ND, normal saline, normal diet; NS-KD, normal saline, ketogenic diet; KA-ND, kainic acid, normal diet; KA-KD, kainic acid, ketogenic diet. |
 | Fig. 4Immunohistochemistry of cathepsin E in rat hippocampus. After i.p. administration of normal saline (A and B) or KA (12 mg/kg in C and D, 25 mg/kg in D, E, G and H), rats were fed a normal diet (A, C, E and G) or the ketogenic diet (B, D, F and H). The CA3 region of the rat hippocampus of the NS-ND (A) and the NS-KD (B) equally showed very few cathepsin-E positive neurons. In the lower-dose KA-ND rat (C), a small number of neurons expressed cathepsin E, while those of the lower-dose KA-KD rat (D) distributed more sparsely. In the CA3 region of the higher-dose KA-ND rat (E), widespread expression of cathepsin E by neurons were observed, in contrast to a marked decrease in the cathepsin E immunoreactivity in the same area of the higher-dose KA-KD rat (F). Details of higher-dose KA-ND neurons exhibit pyknotic changes (G, arrowheads), whereas neurons of the higher-dose KA-KD rat had round, euchromatic nuclei (H, arrowheads). A, B, G, and H were counterstained (scale bar = 500 µm in A, 100 µm in G; A through F are in a same degree of magnification, so are G and H). KA, kainic acid; NS-ND, NS-ND, normal saline, normal diet; NS-KD, normal saline, ketogenic diet; KA-KD, kainic acid, ketogenic diet; CA3, cornu ammonis. |
References
1. Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921. 2:307–308.
2. Huffman J, Kossoff EH. State of the ketogenic diet(s) in epilepsy. Curr Neurol Neurosci Rep. 2006. 6:332–340.

3. Cross JH, Neal EG. The ketogenic diet-update on recent clinical trials. Epilepsia. 2008. 49:Suppl 8. 6–10.

5. Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. Institute Neurology IRCCS C. Mondino Foundation. The ketogenic diet: From molecular mechanisms to clinical effects. Epilepsy Res. 2006. 68:145–180.

6. Pisa M, Sanberg PR, Corcoran ME, Fibiger HC. Spontaneously recurrent seizures after intracerebral injections of kainic acid in rat: a possible model of human temporal lobe epilepsy. Brain Res. 1980. 200:481–487.

7. Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985. 14:375–403.

8. Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injection of kainic acid: a histological study. Neuroscience. 1980. 5:991–1014.

9. Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999. 10:1517–1522.

10. Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, et al. The protective effect of a ketogenic diet on kaninic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003. 53:119–128.

11. Nakanishi H, Tsukuba T, Kondou T, Tanaka T, Yamamoto K. Transient forebrain ischemia induces increased expression and specific localization of cathepsins E and D in rat hippocampus and neostriatum. Exp Neurol. 1993. 121:215–223.

12. Nakanishi H, Tominaga K, Amano T, Hirotsu I, Inoue T, Yamamoto K. Age-related changes in activities and localizations of cathepsins D, E, B, and L in the rat brain tissues. Exp Neurol. 1994. 126:119–128.

13. Amano T, Nakanishi H, Oka M, Yamamoto K. Increased expression of cathepsins E and D in reactive microglial cells associated with spongiform degeneration in the brain stem of senescence-accelerated mouse. Exp Neurol. 1995. 136:171–182.

14. Tominaga K, Nakanishi H, Yasuda Y, Yamamoto K. Excitotoxin-induced neuronal death is associated with response of a unique intracellular aspartic proteinase, cathepsin E. J Neurochem. 1998. 71:2574–2584.

15. Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999. 40:138–143.

16. Su SW, Cilio MR, Sogawa Y, Silveira DC, Homes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Brain Res Dev Brain Res. 2000. 125:131–138.

17. Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H] dopa in various regions of the brain. J Neurochem. 1966. 13:655–669.

18. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001. 29:e45.

19. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006. 60:223–235.
20. Tai KK, Truong DD. Ketogenic diet prevents seizure and reduces myoclonic jerks in rats with cardiac arrest-induced cerebral hypoxia. Neurosci Lett. 2007. 425:34–38.
21. Kim do Y, Rho JM. The ketogenic diet and epilepsy. Curr Opin Clin Nutr Metab Care. 2008. 11:113–120.
22. Zaidi N, Hermann C, Herrmann T, Kalbacher H. Emerging functional roles of cathepsin E. Biochem Biophys Res Commun. 2008. 377:327–330.

23. Bernstein HG, Wiederanders B. An immunohistochemical study of cathepsin E in Alzheimer-type dementia brains. Brain Res. 1994. 667:287–290.

24. Gasior M, Rogawaski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006. 17:431–439.

26. Willmore LJ. Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav. 2005. 7:Suppl 3. S25–S28.
27. Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav. 2005. 7:Suppl 3. S3–S11.

28. Meller R, Schindler CK, Chu XP, Xiong ZG, Cameron JA, Simon RP, et al. Seizure-like activity leads to the release of BAD from 14-3-3 protein and cell death in hippocampal neurons in vitro. Cell Death Differ. 2003. 10:539–547.

29. Noh HS, Kim YS, Kim YH, Han JY, Park CH, Kang AK, et al. Ketogenic diet protects the hippocampus from kainic acid toxicity by inhibiting the dissociation of bad from 14-3-3. J Neurosci Res. 2006. 84:1829–1836.

30. Matarrese P, Straface E, Pietraforte D, Gambardella L, Vona R, Maccaglia A, et al. Peroxynitrite induces senescence and apoptosis of red blood cells through the activation of aspartyl and cysteinyl protease. FASEB J. 2005. 19:416–418.
31. Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, et al. Cathespin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007. 67:10869–10878.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download