Abstract
Purpose
The purpose of this study is to construct a recombinant adenovirus vector carrying mouse 4-1BBL and observe its effects in dendritic cells.
Materials and Methods
Mouse 4-1BBL cDNA was taken from the plasmid pcDNA3-m4-1BBL and subcloned into adenovirus shuttle plasmid pAdTrack-CMV, and then transformed into competent BJ5183 with plasmid pAdEasy-1. After recombination in E. coli, Ad-4-1BBL was packaged and amplified in HEK 293 cells. The expression of 4-1BBL in Ad-4-1BBL-transfected mouse prostate cancer cell line RM-1 was detected by reverse transcription polymerase chain reaction (RT-PCR) and Western blot. After the co-culture of dendritic cells (DCs) with Ad-4-1BBL-transfected RM-1 cells, interleukin (IL)-6 and IL-12 production were assessed by enzyme-linked immunosorbent assay (ELISA) and co-stimulatary moleculs (CD80 and CD86) on DCs were analyzed by flow cytometry.
Results
The levels of IL-6 (3,960 pg/mL) and IL-12 (249 pg/mL) production in Ad-m4-1BBL-pulsed DCs were more than those in none-pulsed DCs. The differences were statistically significant (p < 0.05). The expression of co-stimulatary molecules (CD80 and CD86) was up-regulated in Ad-m4-1BBL-pulsed DCs.
Dendritic cells (DCs) are potent antigen-presenting cells that play a central role in immunity.1 DC capture and process antigen are efficient and adept at stimulating T cells and NK cells. The level of maturation is an important element of DC function. Immature DCs are efficient at capturing antigen while they are relatively poor at presenting antigen to T cells. Conversely, mature DC do not capture antigen as well but potently-stimulated T cells. Maturation is a terminal and critical event in DC development. DC require maturation before exhibiting their full immuno-stimulatory and antitumor potential.
It has been shown that 4-1BBL is expressed on mature B cells, macrophages, and dendritic cells.2 4-1BBL is a member of the tumor necrosis factor (TNF) superfamily of type II membrane protein. 4-1BBL with its receptor 4-1BB expressed on T interaction provided a co-stimulatory signal to T cells, including activation of both CD4+ and CD8+ T cells, enhanced expansion,3,4 increased long-term survival,5,6 and anti-apoptosis of activation-induced CD8+ T cells.7 Costimulation through 4-1BBL/4-1BB can also promote enhanced production of cytokines such as interleukin (IL)-2, IL-4, and interferon (IFN)-λ.3,8 Futagawa, et al.9 have shown that immature DC expressed 4-1BBL at a high level and the 4-1BB-4-1BBL interaction represent a novel pathway of DC activation by DC-DC interaction.
To further characterize the function of 4-1BBL in DC activation, we used replication-deficient E1a-E1b/E3 deletion mutant adenovirus sero-type 5 to yield the adenovirus-4-1BBL (Ad-4-1BBL), and detected the change of immunoactivity of dendritic cells induced by Ad-m4-1BBL.
Female C57BL/6 (H-2 Kb) mice, 6-8 wk old, were obtained from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). Animals were maintained at the Central Animal Facility of Wuhan University according to standard guidelines and experiments were conducted according to the guidelines of the China Council for Animal Care. RM-1, a murine prostate cancer cell line, was obtained from the Chinese Academy of Sciences (Shanghai, China). HEK 293, human embryonic kidney 293 cell line, was provided by the Ministry of Education Key Laboratory of Virologyo (Wuhan, China). All cells were cultured in RPMI-1640 medium with 10% fatal calf serum (FCS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37℃ in a humidified atmosphere containing 5% CO2.
The E1 and E3 regions deficient serotype 5 recombinant adenovirus vector was used to construct Ad-m4-1BBL using pAdEasy-1 system gifted by the Ministry of Education Key Laboratory of Virologyo (Wuhan, China). Briefly, the murine 4-1BBL gene was amplified from plasmid pcDNA3-m4-1BBL gifted by Dr. Tania Watts (Department of Immunology, University of Toronto, Canada). The amplified DNA fragment was inserted into the shuttle vector pAdTrack-CMV in the BglII/HindIII-restriction sites. The resultant plasmid was linearized by PmeI and subsequently cotransformed into E. coli BJ5183 with a adenoviral backbone plasmid (pAdEasy-1). Then the recombinant adenoviral plasmid (pAd-m4-1BBL) was transfected into HEK 293 cells by Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) for amplification. Adenovirus was purified by centrifugation in a cesium chloride gradient. The Ad-eGFP was constructed similarly using for control Ad virus.
RM-1 cells (1×106) were infected with Ad-4-1BBL [multiplicity of infection (MOI) 50]. Two days later, cells were collected and total ribonucleic acid (RNA) was extracted using the Trizol agent. One microgram of total RNA was used as a template for complementary DNA (cDNA) synthesis using random primers and River Tra Ace according to the manufacturer's instructions. Following amplification by polymerase chain reaction (PCR), the cycling conditions are as follows: predegeneration at 94℃ for 5 min, denaturation at 94℃ for 30s, annealing at 55℃ for 30s, and extension at 72℃ for 45s for tPSMA (30 cycles), 4-1BBL (30 cycles), and β-Actin (25 cycles). 4-1BBL forward primer F was: 5'-TGTTCGCCAAGCTACTG-3', reverse primer R was: 5'-ATGGGTTGTCGGGTTTCA-3', β-actin forward primer F was: 5'-TCATGAAGT GTGACGTGGACATC-3', and reverse primer R was: 5'-CAGGAGGAGCAATGATCTTGATCT-3'. After amplification, the PCR products were resolved by 1.2% agarose gel electrophoresis.
RM-1 cells (1×106) were infected with the Ad-m4-1BBL for 24 h, scraped and lysed. The lysates were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoress (SDS-PAGE). After electrophoresis, the protein blots were transferred to a nitrocellulose membrane (Amersham, Waukesha, WI, USA). The membrane was blocked with 5% non-fat milk in TBST for 1 h and incubated overnight with rabbit anti-4-1BBL polyclonal Ab at 4℃. After three washes with TBST, the membrane was incubated at 37℃ for 1 h with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibody diluted with TBST (1 : 1000). The detected protein signals were visualized by an enhanced chemilluminescence reaction system. Western blot for β-Actin was used as an internal sample.
Mouse DCs were generated from bone marrow suspensions harvested from 6-8 week old C57BL/6 mice according to the publication10 with slight modifications. Briefly, bone-marrow cells were harvested from femurs and tibias, depleted of red blood cells, and washed twice in phosphate buffered saline (PBS). Cells were resuspended in a DC medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco, NY, USA), 10 ng/mL GM-CSF (R&D Systems, Minneapolis, MN, USA), 10 ng/mL IL-4 (R&D Systems, Minneapolis, MN, USA), and 50 mM 2-mercaptoethanol, 100 IU/mL penicillin, and 100 µg/mL streptomycin and cultured (37℃, 5% CO2) in 6-well plates at 1×106 cells/3 mL/well. On day 3 and 5 of culture, floating cells were gently removed, and fresh mGM-CSF/mIL-4-containing medium was added. On day 6, non-adherent cells and loosely adherent proliferating BMDCs aggregates were collected.
DCs co-culture was performed with three types of RM-1 cells (Ad-4-1BBL-tranduced RM-1 cells, Ad-eGFP-tranduced RM-1 cells, and normal RM-1 cells control) as stimulator cells and DCs as responder cells. Stimulator cells were incubated with Mitomycin C (MMC) at 50 ng/mL at 37℃ for 30 min and then washed with PBS twice. DCs were plated in 24-well round-bottomed culture plate (Costar, Union, CA, USA) at 4×105 cells per well. Then, 2×105 stimulators were added and co-cultured with responders after additional 48 h in a complete RPMI-1640 medium with 10 ng/mL GM-CSF and 10 ng/mL IL-4.
For phenotypic analyses by flow cytometry, DCs were stained for 30 min on ice with FITC- or PE-labeled monoclonal antibodies specific for CD11c, CD80, and CD86 (BD Pharmingen, San Diego, CA, USA), after washed three times in PBS, the cells were analyzed by flow cytometry. Isotype-matched monoclonal antibodies were used as controls.
For cytokine assays, co-culture supernatants were harvested and used for enzyme-linked immunosorbent assay (ELISA). A mouse IL-6 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) and a mouse IL-12 Quantikine ELISA Kit (R&D Systems) were used to detect IL-6 and IL-12, respectively, following the manuacturer's instructions.
The pAdTrack-CMV-4-1BBL containing full-length 4-1BBL cDNA was detected by BglII and HindIII. Two specific bands represented the pAdTrack-CMV shuttle vector and 4-1BBL (Fig. 1). Then, the resultant pAdTrack-CMV-4-1BBL was linearized with pmeI and cotransformed with pAdEasy-1 backbone vector into competent BJ5183. Recombinants were screened by restriction endonuclease digestion with PacI (Fig. 2).
RM-1 cells were infected with recombinant adenovirus for 48 hours. Cell protein was extracted to perform Western blot analysis. Western blot analysis showed the 4-1BBL protein expressed in cells infected with Ad-4-1BBL, but nothing in the control cells (Fig. 4). β-actin protein were detected in all samples.
To determine whether mouse 4-1BBL recombinant adenovirus effected co-stimulatory molecules on DCs, bone marrow-derived DCs co-cultured with RM-1 cells infected with Ad-4-1BBL, Ad-eGFP or uninfected, were analyzed after costaining for CD11c and CD80, CD86 (Fig. 5). The results indicate that DCs co-cultured with RM-1 cells infected with Ad-4-1BBL specifically expressed high levels of CD80 and CD86.
To determine the mechanism of affection of DCs induced by 4-1BB/4-1BBL costimulation, we analyzed cytokine production by BMDC and RM-1 cells. Bone marrow-derived DCs co-cultured with RM-1 cells infected with Ad-4-1BBL, Ad-eGFP or uninfected. After 48 h, the culture supernatants were harvested and analyzed for the production of IL-6 and IL-12 by ELISA. The level of IL-6/IL-12 in Ad-4-1BBL-treated supernatant was more than the other two groups. (Fig. 6).
DCs are one of the most potent APCs for the induction of antitumor immune responses currently known and have been recognized as potentially important tools for cancer vaccine strategies.1 One of the important elements of DCs function is the level of their maturation. The mature DCs expressed 4-1BBL, with which 4-1BB interaction could enhance the maturation of DCs and the activation of T cells.10 In this study, we used replication-deficient adenovirus to generated a recombinant adenovirus encoding 4-1BBL (Figs. 1 and 2), because of the adenovirus is the most efficient for gene transfer. We confirmed the expression of 4-1BBL in Ad-4-1BBL-transfected RM-1 cells (Figs. 3 and 4), and demonstrated that 4-1BBL induced pro-found changes in DC immune properties. DCs cocultured with Ad-4-1BBL-transfected RM-1 cells produced higher levels of IL-6/IL-12 than Ad-eGFP-transfected and non-transfected RM-1 cells; the difference was significant (p < 0.01) (Fig. 6). Ad-4-1BBL-transfected RM-1 cells induced CD80/ CD86 up-regulation on DCs (Fig. 5).
In this study, we further confirmed the expression of 4-1BB on DC, and the 4-1BB molecules expressed on DC were functional to active DC. Our results were consistent with previous reports,9 which showed that the 4-1BB/4-1BBL interaction could induce DC activation by DC-DC interaction. Recent reports have shown that DC play a important role in eliciting anti-tumor immunity.11,12 However, mechanisms for the DC-tumor interaction remain largely unknown. Our present results suggest a possibility that 4-1BB molecules expressed on DC may be involved in tumor surveillance by DC. It should be also noted that the 4-1BBL-mediated activation of DC might be involved in the anti-tumor effect of 4-1BBL gene transfer. This modality has been thought to exert the immunopotentiating effect by directly co-stimulating T cells. However, our results suggested that DC activation by this modality might play a rather critical role.
Recent studies using Ad-4-1BBL detect the change of immunoactivity of dendritic cells. However, the mechanisms for the DC-tumor interaction need to further investigation.
Figures and Tables
Fig. 1
Identification of pAdTrack-CMV-4-1BBL by restriction endonucleases digestion. M. λ-EcoT14 DNA Marker; 1, pAdTrack-CMV digested with Bgl II; 2 and 3, pAdTrack-CMV-m4-1BBL digested with Bgl II/Hind III.
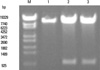
Fig. 2
Analysis of pAd-m4-1BBL by PacI. 1, 2 and 3, pAd-m4-1BBL digested with PacI; M. λ/Hind III DNA Marker.

Fig. 3
Detection of 4-1BBL mRNA by RT-PCR. RM-1 cells were transfected with pAd-4-1BBL. Total RNA was extracted, and 4-1BBL mRNA was detected by RT-PCR. (A) M, DL 2000 DNA marker; 1, RM-1 cells transfected with pAd-4-1BBL; 2, RM-1 cells. (B) β-actin was used as reference. mRNA, messenger ribonucleic acid, RT-PCR, reverse transcription polgmerase chain reaction.
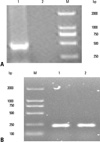
Fig. 4
Detection of 4-1BBL protein by Western blot. Total cell lysates were harvested and presence of 4-1BBL protein was detected by anti-4-1BBL polyclonal antibody. A specific band was identified in RM-1 cells transfected with Ad-4-1BBL but not in none-transfected RM-1 cells. β-actin was used as reference.
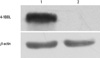
Fig. 5
Activation of DCs by 4-1BBL. Bone-marrow-derived DCs from C57BL/6 mice were co-cultured with non-transfected, Ad-eGFP-transfected or Ad-4-1BBL-transfected RM-1 cells. Flow cytometry showed expression of CD80, CD86 molecules was significantly increased 4-1BBL. The experiment was repeated three with representative data shown. DCs, dendritic cells.

Fig. 6
Ad-4-1BBL-transfected RM-1 cells induce IL-6/IL-12 production by DCs. DCs were co-cultured with Ad-eGFP-transfected, Ad-4-1BBL-transfected or none-transfected RM-1 cells. Cell-free supernatants were collected at 48 h and IL-6/IL-12 were measured by Elisa. (A) The level of IL-6 in DC/Ad-4-1BBL group was more than that in DC and DC/Ad-eGFP group, *p < 0.05. (B) The production of IL-12 in DC/Ad-4-1BBL group was higher than that in DC and DC/Ad-eGFP group, *p < 0.05. Data are expressed as mean ± SD. Similar results were obtained from three independent experiments. IL, interleukin; SD, standard deviation.

ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 30672107, 30901494, 30901552). A special thanks to Dr. Tania Watts (Department of Immunology, University of Toronto, Canada) for providing pcDNA3-m4-1BBL and Dr. Jianguo Wu (Ministry of Education Key Laboratory of Virologyo, Wuhan University).
References
1. Nencioni A, Grünebach F, Schmidt SM, Müller MR, Boy D, Patrone F, et al. The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol. 2008. 65:191–199.
2. Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol. 2005. 77:281–286.
3. Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol. 2002. 14:1155–1167.

4. Lu ZY, Condomines M, Tarte K, Nadal L, Delteil MC, Rossi JF, et al. B7-1 and 4-1BB ligand expression on a myeloma cell line makes it possible to expand autologous tumor-specific cytotoxic T cells in vitro. Exp Hematol. 2007. 35:443–453.

5. Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002. 169:4882–4888.

6. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003. 3:609–620.
7. Kudo-Saito C, Hodge JW, Kwak H, Kim-Schulze S, Schlom J, Kaufman HL. 4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines. Vaccine. 2006. 24:4975–4986.
8. Xiao H, Huang B, Yuan Y, Li D, Han LF, Liu Y, et al. Soluble PD-1 facilitates 4-1BBL-triggered antitumor immunity against murine H22 hepatocarcinoma in vivo. Clin Cancer Res. 2007. 13:1823–1830.

9. Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002. 14:275–286.

10. Wiethe C, Dittmar K, Doan T, Lindenmaier W, Tindle R. Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen. J Immunol. 2003. 170:2912–2922.

11. Nencioni A, Grünebach F, Schmidt SM, Müller MR, Boy D, Patrone F, et al. The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol. 2008. 65:191–199.

12. Hu Y, He Y, Srivenugopal KS, Fan S, Jiang Y. In vitro antitumor cytotoxic T lymphocyte response induced by dendritic cells transduced with DeltaNp73alpha recombinant adenovirus. Oncol Rep. 2007. 18:1085–1091.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download