Abstract
Purpose
The purpose of this paper is to test the hypothesis that combination therapy of serial cast and botulinum toxin type A (BTX-A) injection can further enhance the effects of a BTX-A injection in ambulant children with cerebral palsy (CP) who have an equinus foot.
Materials and Methods
Children in group A (30 legs of 21 children) received a serial casting application after an injection of BTX-A, and children in group B (25 legs of 17 children) received only a BTX-A injection. Assessments were performed before the intervention and 1 month after the intervention.
Results
After the intervention, there were significant improvements in tone, dynamic spasticity, and passive range of motion (ROM) in both groups. However, the changes were greater in group A than in group B. Dimension D (standing) in Gross Motor Function Measure (GMFM)-66 was significantly improved in group A but not in group B. On the other hand, there were no significant changes in di-mension E (walking, running, jumping) in GMFM-66 in either group.
Equinus is the most common problem in ambulatory children with spastic cerebral palsy (CP), which results in an unstable and inefficient gait pattern. Without proper management at an early stage, it can evolve into permanent foot deformities that may require multiple surgical interventions. Early therapeutic intervention to address this problem can allow children to maintain or regain full range of motion (ROM) and maximize functional mobility. In addition, such an intervention may help to prevent contracture or to postpone surgical intervention. Currently, a number of conservative interventions for managing equinus in children with CP are available, such as bracing, stretching exercises, electrical stimulation, and casts/serial casting.1-3 Botulinum toxin type A (BTX-A) injection into triceps surae muscles has been considered an effective therapeutic approach. Reduction of tone and an increase in ROM at the ankle joint and an improvement of gait pattern with a BTX-A injection have been demonstrated in literature.2,4-7
The immobilization of a muscle in a lengthened position for a prolonged period of time gradually increases in both length and the number of sarcomeres in the target muscle.8 According to a previous study,9 a spastic muscle responds to progressive casting by increasing its length in the same way that a normal muscle responds. Thus, casting in children with CP has been used to increase ROM10 through this physiological adaptation to prolonged stretching. A significant increase in ROM and tone reductions with casts in children with CP were reported in previous studies.1,2,11-14 Compared to a single fixed cast, serial casting involves the removal and reapplication of a cast at certain intervals. Each time the cast is changed, the ankle is positioned into greater dorsiflexion in order to stretch the calf muscle progressively up to the desired ROM at the ankle joint. When a mild fixed ankle plantarflexor contracture is present, serial casting seems to be effective in lengthening the triceps surae musculature.
Both BTX-A injection and casting seem to be effective for reducing tone and improving ROM. The effects of both modalities for managing equinus in these children were compared to those in previous studies. Various results showing similar efficacy for both modalities15 or favoring BTX-A injecton2 or favoring casting13,16 were reported in previous studies. Moreover, only a few reports show the effects of the combined therapy of BTX-A injection and casting for managing equinus in these children.13,14,16-18 While in some studies combined therapy resulted in better outcomes than those of BTX-A alone,13,16,17 better outcomes were reported with serial casting alone than the combined therapy of BTX-A and serial casting.14 Therefore, the objective of the present study was to determine whether the combined therapy of serial casting and BTX-A injection can further enhance the effects of the BTX-A injection in ambulant children with CP who have equinus foot.
Among children with spastic CP, the children who met the following inclusion criteria were selected as subjects for this study: 1) able to walk independently without aids, 2) had an equinus foot with mild shortening of the calf muscle that does not allow the ankle with knee extended to dorsiflex more than 10° , 3) had not yet developed joint or bone deformities, and 4) had not had orthopaedic surgery before. In total, 38 children were recruited as participants for this study. According to each parent's willingness to have his or her child undergo serial casting, serial casting was applied to 30 legs of 21 children after BTX-A (group A). BTX-A alone was administered to 25 legs of 17 children (group B).
This study was conducted with the approval of our Institutional Review Board (4-2008-0456).
The muscle tone of the ankle plantar flexor with the knee in flexion and extension was assessed with the modified Ashworth scale (MAS), which is a 6-point rating scale from 0 to 4 used to gauge muscle tone.19 For the convenience of statistical analysis, MAS 1 + was converted to 2. Likewise, MAS 2, 3, and 4 were converted into 3, 4 and 5, respectively. In addition, the modified Tardieu scale (MTS) was used to assess the ankle plantar flexor with the knee in flexion and extension.20 Two levels of passive ROMs were measured, referring to R1 and R2 angles. R2 refers to total passive ROM into ankle dorsiflexion, while R1 refers to the point in the ROM where a 'catch' was felt during a quick stretch of the ankle plantarflexor. The ankle ROM angle in MTS was measured by manual goniometry with the "neutral-null" method (dorsiflexion angle over the neutral position was counted in positive degrees, under the neutral in negative degrees). Gross Motor Function Measure (GMFM)-66, which has excellent reliability (ICC = 0.99) and is responsive to change, was used to assess motor function.21 It is divided into five dimensions of motor function (A: lying and rolling, B: sitting, C: crawling and kneeling, D: standing, and E: walking, running, jumping).22 All of the children were able to walk independently, and therefore dimensions D and E in GMFM-66 were chosen as functional measures for this study.23 The assessment was performed by a physical therapist for GMFM scoring and by a physician for other outcome measures who were unaware of the type of intervention that the child had received. All of the parameters were recorded the before intervention and at 1 month after the intervention.
BTX-A was injected into both the medial and lateral heads of spastic gastrocnemius muscle. Doses selected for BTX-A injection were individualized on the basis of body weight, severity of spasticity, and movement pattern as established by our empirical methods. Before injection, a lidocaine-based local anesthetic, EMLA cream (Astra, Kings Langley, UK), was applied to the skin and a sample of BTX-A (Botox®; Allergan, Inc, Irvine, CA, USA) diluted with 2 mL of 0.9% saline was injected into the target muscles under the guidance of ultrasonography.
Serial casting consisted of short leg casts applied in prone position with the knee flexed to 90° and the ankle dorsiflexed to maximal attainable dorsiflexion and held in the neutral hind foot position. Three consecutive casts were applied for 1 week each with a progressively increasing amount of ankle dorsiflexion to stretch the calf muscle. Ankle-foot orthosis was applied to the children in group B immediately after the injection and after the completion of serial casting in group A.
The intensity and frequency of physical and occupational therapy given to the children after the intervention was the same for both groups. Both groups underwent 30 minutes of physical therapy and 30 minutes of occupational therapy per day six times a week. Physical and occupational therapy comprised play-based activities based on the neurodevelopmental techniques. Physical therapy focused to improve performance in and gain independence with such tasks as making transfers, stretching, bed mobility, and safe ambulation. Occupational therapy focused on tasks such as weight bearing on the arm, reaching, grasping, holding, manipulating an object, fine motor hand skills, and activities of daily living that were age appropriate.
SAS version 9.1.3 was used for statistical analyses. Between groups A and B, the numerical parameters were compared using an independent t-test, and the nominal data were compared using a Chi-square test. In each group, the parameters before and after treatment were compared using a paired t-test. Statistical significance was defined as a p-value of < 0.05.
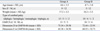
The characteristics of participants and the values of parameters assessed before the intervention are described in Table 1. The mean age of the children was 4.6 years (range 2.3 to 8.3 years) in group A and 4.7 years (range 3.6 to 7.8 years) in group B. There were no significant differences in age, body weight, the distributions of sex, and the functional levels based on the Gross Motor Function Classification System (GMFCS) between the groups (p > 0.05) (Table 1). The mean dose of BTX-A injected into each gastrocnemius was 4.9 (range 4.0 to 6.0) units/kg and 5.0 (range 4.5 to 6) units/kg in groups A and B, respectively. There was no significant difference in the doses of BTX-A between the groups.
In addition, the baseline measurements of the MAS and the R1 and R2 angles in the MTS were not significantly different between the groups (p > 0.05). The scores of dimension D and E in GMFM-66 in group A were not significantly different from those in group B (p > 0.05) (Table 1).
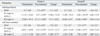
The MAS of the ankle joint with knee flexion and extension was significantly reduced in both groups after intervention (p < 0.05). The MAS at the ankle joint with knee flexion after intervention was significantly lower in group A than in group B (p < 0.05), whereas the MAS was not significantly different between the two groups at the ankle joint with knee extension. Significant differences in the changes of MAS between the two groups were noted at the ankle joint only with knee flexion, but not with knee extension (Table 2).
R1 angles of MTS at the ankle joint with knee flexion and extension significantly improved after treatment in both groups (p < 0.05). The R1 angles in group A were significantly higher at the ankle joint with knee flexion and extension than those in group B (p < 0.05). Furthermore, the changes were greater in group A than in group B at the ankle joint with knee flexion and extension (p < 0.05) (Table 2).
The R2 angles of MTS at the ankle joint with knee flexion and extension significantly increased after intervention in both groups (p < 0.05). The changes at both knee flexion and extension were significantly greater in group A than in group B (p < 0.05) (Table 2).
In group A, there was significant improvement in dimension D of GMFM-66 after treatment (p < 0.05), but not in dimension E of GMFM-66 (p > 0.05). In group B, there were no significant changes in dimension D and E of GMFM-66 after treatment (p > 0.05) (Table 3).
An equinus foot in children with spastic CP is caused by spasticity or shortening of the gastocnemius-soleus muscle complex or a combination of these two components. Initially, it appears to be a dynamic phenomenon, secondary to a central nervous system lesion. As the child grows, the progressive shortening of the calf muscles secondary to spasticity develop to such a significant degree that the contracture may require surgical lengthening of the muscle or tendon unit. For dynamic equinus, a BTX-A injection into the calf muscles has been considered an effective form of management.2,4,5 For such cases, BTX injection into the triceps surae muscles results in significant tone reduction and an increase in ROM has been noted in literature.5-7 Our present study confirms the findings of previous reports on the efficacy of BTX-A injections in the treatment of equinus in children with CP.
Since BTX-A injection works mainly on spasticity, the effect of BTX-A injection is limited when there is muscle contracture. On the other hand, casting works mainly on muscle contracture, and therefore, cast application might be helpful for gaining additional ROM in cases in which there is muscle contracture.13,24 In particular, two kinds of cast application were used in the literature for managing equinus in the children with CP. A single fixed cast is applied at the neutral position of the ankle joint for a certain period of time, while serial cast applications involve removals and reapplications at certain intervals to ensure more ankle dorsiflexion at each cast. In five previous studies showing the effects of combined therapy of cast and BTX-A, a single fixed cast was used in two reports,16,17 while serial casts were used in three studies.13,14,18 In the reports using serial casts in conjunction with BTX-A, more marked improvement in passive ankle dorsiflexion was achieved in 2 reports,13,14 and faster improvements in ROM18 were achieved, compared to treatment with BTX-A alone. In reports using a single fixed cast, the improvement in ROM was greater after treatment with combined therapy than BTX-A alone.16 On the other hand, the addition of a fixed cast to BTX-A did not result in any significant gains in the passive ROM of the ankle joint in the report by Bottos, et al.17 Since the effects of serial casting on passive ROM have not yet been studied, in comparison with a single fixed cast for treating equinus in these children, it is not yet known whether serial casts or fixed casts are more effective.25 However, theoretically, the serial cast may be more effective in lengthening the shortened musculature than a single fixed cast. Additional gains in the ROM in the group receiving both BTX-A and serial casting in our study likely support the hypothesis that serial casting is an effective therapeutic approach for improving ankle passive ROM.
On the other hand, in terms of tone changes, two reports revealed better responses in tone reduction with combined therapy of a single fixed cast and BTX-A, compared to BTX-A alone.16,17 Of the two reports using serial casting applications, changes in tone were described only in the report by Kay, et al.,14 in which serial casting alone led to better responses compared to combined therapy of serial casting and BTX-A. Despite the results of their study, they suggested that the benefits of adding BTX-A injection to serial casting on maximizing the tolerance of the casting may outweigh the risk of early recurrence of spasticity in patients with more spasticity or dystonia. These previous studies seem likely to support that combined therapy with BTX-A and cast application benefits tone reduction. The present study showed that the addition of serial casting to BTX-A injection can result in further benefits in tone reduction compared to BTX-A alone. Because we did not evaluate the sample size, we also calculated the observed statistical power. The observed power of the change in R1 and R2 angles of MTS at the ankle joint with extension was 0.72 and 0.79, respectively. We thought that more cases would be needed for a larger statistical power. Further study will be needed.
In addition, changes in passive and dynamic ankle motion with combination therapy seem to result in significant improvement in dimension D (standing) in GMFM-66. However, there was no significant change in dimension E (walking) in GMFM-66. Activities in dimension E are more difficult to achieve than those of dimension D. Hence, several exercises are required to produce any significant changes in dimension E. This might explain why significant change was achieved only in dimension D, but not dimension E in our short-term follow-up. In a previous study,17 the GMFM-66 (standing and walking scores) scores significantly improved 4 months after the intervention of BTX-A with a single cast; therefore, we think that dimension E (walking) may improve in the long-term assessment.
As for the longevity of the effects of casting application for the equinus of children with CP, some studies revealed long-lasting changes throughout the 12-month follow-up period,14,16 while others showed much shorter-lasting effects.2,3 A single fixed cast application in conjunction with a BTX-A injection, in comparison with BTX-A alone, resulted in more enduring effects in the reports by Bottos, et al.17 and Ackman, et al.16 In contrast, the addition of BTX to serial casting revealed shorter-term effects, compared to serial casting alone, in cases of fixed contracture in the report by Kay, et al.14 With our short-term follow-up study, we could not determine the time when the effects of the interventions started to deteriorate because the follow-up periods in our cases varied. A long-term follow up study is needed to find out whether additional serial casting applications after BTX-A injection can lead to more functional benefits and more enduring results.
Our study was limited in that we did not perform formal reliability testing for the assessments measured in this study. However, all range of motion and spasticity measurements were performed with the use of consistent techniques by the same physician (W.H.C.), and the GMFM tests were performed with the use of a standardized procedure and scoring22 by the same physical therapist (Y.L.) who had more than 5 years of experience. In addition, a previous report revealed high inter-rater reliability, ranging from 0.85 to 0.96 in these measures assessing the outcomes of the interventions to treat equinus in the children with CP. In addition, they concluded that, with regard to acceptability, practicability, and responsiveness, these tests could be effectively performed on these children who have equinus deformity.26 As a result, we believe that the changes observed in our study with these assessments are acceptable and reliable.
In conclusion, significant improvements in tone and ROM with BTX-A alone suggest that BTX-A injection may be effective in treating a spastic equinus foot in children with CP. On the other hand, children who underwent both serial casting and BTX-A injection showed more marked changes in tone reduction, dynamic ankle motion, and passive ROM. In addition, dimension D in GMFM-66 significantly improved in the combination therapy group. These findings suggest that combination therapy of BTX-A and serial casting is a promising therapeutic strategy for enhancing the benefits of BTX-A injection in children with CP. However, a long-term follow up study is needed to find out whether additional serial casting applications after BTX-A injection can lead to more functional benefits and more enduring results.
Figures and Tables
References
1. Brouwer B, Davidson LK, Olney SJ. Serial casting in idiopathic toe-walkers and children with spastic cerebral palsy. J Pediatr Orthop. 2000. 20:221–225.
2. Corry IS, Cosgrove AP, Duffy CM, McNeill S, Taylor TC, Graham HK. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. J Pediatr Orthop. 1998. 18:304–311.
3. McNee AE, Will E, Lin JP, Eve LC, Gough M, Morrissey MC, et al. The effect of serial casting on gait in children with cerebral palsy: preliminary results from a crossover trial. Gait Posture. 2007. 25:463–468.

4. Galli M, Cimolin V, Valente EM, Crivellini M, Ialongo T, Albertini G. Computerized gait analysis of botulinum toxin treatment in children with cerebral palsy. Disabil Rehabil. 2007. 29:659–664.

5. Sätilä H, Pietikäinen T, Iisalo T, Lehtonen-Räty P, Salo M, Haataja R, et al. Botulinum toxin type A injections into the calf muscles for treatment of spastic equinus in cerebral palsy: a randomized trial comparing single and multiple injection sites. Am J Phys Med Rehabil. 2008. 87:386–394.

6. Bang MS, Chung SG, Kim SB, Kim SJ. Change of dynamic gastrocnemius and soleus muscle length after block of spastic calf muscle in cerebral palsy. Am J Phys Med Rehabil. 2002. 81:760–764.

7. Papadonikolakis AS, Vekris MD, Korompilias AV, Kostas JP, Ristanis SE, Soucacos PN. Botulinum A toxin for treatment of lower limb spasticity in cerebral palsy: gait analysis in 49 patients. Acta Orthop Scand. 2003. 74:749–755.

8. Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon). 2001. 16:87–101.

9. Tardieu G, Tardieu C, Colbeau-Justin P, Lespargot A. Muscle hypoextensibility in children with cerebral palsy: II. Therapeutic implications. Arch Phys Med Rehabil. 1982. 63:103–107.
10. Desloovere K, Molenaers G, Jonkers I, De Cat J, De Borre L, Nijs J, et al. A randomized study of combined botulinum toxin type A and casting in the ambulant child with cerebral palsy using objective outcome measures. Eur J Neurol. 2001. 8:Suppl 5. 75–87.

11. Brouwer B, Wheeldon RK, Stradiotto-Parker N, Allum J. Reflex excitability and isometric force production in cerebral palsy: the effect of serial casting. Dev Med Child Neurol. 1998. 40:168–175.
12. Cottalorda J, Gautheron V, Metton G, Charmet E, Chavrier Y. Toe-walking in children younger than six years with cerebral palsy. The contribution of serial corrective casts. J Bone Joint Surg Br. 2000. 82:541–544.
13. Glanzman AM, Kim H, Swaminathan K, Beck T. Efficacy of botulinum toxin A, serial casting, and combined treatment for spastic equinus: a retrospective analysis. Dev Med Child Neurol. 2004. 46:807–811.

14. Kay RM, Rethlefsen SA, Fern-Buneo A, Wren TA, Skaggs DL. Botulinum toxin as an adjunct to serial casting treatment in children with cerebral palsy. J Bone Joint Surg Am. 2004. 86-A:2377–2384.
15. Flett PJ, Stern LM, Waddy H, Connell TM, Seeger JD, Gibson SK. Botulinum toxin A versus fixed cast stretching for dynamic calf tightness in cerebral palsy. J Paediatr Child Health. 1999. 35:71–77.

16. Ackman JD, Russman BS, Thomas SS, Buckon CE, Sussman MD, Masso P, et al. Comparing botulinum toxin A with casting for treatment of dynamic equinus in children with cerebral palsy. Dev Med Child Neurol. 2005. 47:620–627.
17. Bottos M, Benedetti MG, Salucci P, Gasparroni V, Giannini S. Botulinum toxin with and without casting in ambulant children with spastic diplegia: a clinical and functional assessment. Dev Med Child Neurol. 2003. 45:758–762.

18. Booth MY, Yates CC, Edgar TS, Bandy WD. Serial casting vs combined intervention with botulinum toxin A and serial casting in the treatment of spastic equinus in children. Pediatr Phys Ther. 2003. 15:216–220.

19. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987. 67:206–207.

20. Mackey AH, Walt SE, Lobb G, Stott NS. Intraobserver reliability of the modified Tardieu scale in the upper limb of children with hemiplegia. Dev Med Child Neurol. 2004. 46:267–272.

21. Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther. 2000. 80:873–885.

22. Russell DJ. Gross motor function measure (GMFM-66 and GMFM-88) user's manual. 2002. London: Mac Keith Press.
23. Koman LA, Mooney JF 3rd, Smith BP, Goodman A, Mulvaney T. Management of spasticity in cerebral palsy with botulinum-A toxin: report of a preliminary, randomized, double-blind trial. J Pediatr Orthop. 1994. 14:299–303.

24. Jain S, Mathur N, Joshi M, Jindal R, Goenka S. Effect of serial casting in spastic cerebral palsy. Indian J Pediatr. 2008. 75:997–1002.

25. Blackmore AM, Boettcher-Hunt E, Jordan M, Chan MD. A systematic review of the effects of casting on equinus in children with cerebral palsy: an evidence report of the AACPDM. Dev Med Child Neurol. 2007. 49:781–790.
26. Kelly B, MacKay-Lyons MJ, Berryman S, Hyndman J, Wood E. Assessment protocol for serial casting after botulinum toxin a injections to treat equinus gait. Pediatr Phys Ther. 2008. 20:233–241.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download