Abstract
Purpose
Deciding on treatment carcinoma of the tongue when the tumor has a thickness of 1.5 cm or more is difficult. Surgery often requires wide resection and re-construction, leading to considerable functional impairment. A cesium implant is an attractive option, but according to the Manchester System, a two plane implant is needed.
Materials and Methods
According to the textbook, a tumor is sandwiched between the needles, which are implanted at the edge of the tumor. This may cause an unnecessarily high dose to the outer surface of the tongue, which sometimes leads to a persistent ulcer. To avoid this complication, we invented a modified implantation method, and applied the method to five consecutive patients.
Carcinoma of the mobile tongue can be successfully treated by surgery or radiotherapy provided that the tumor is small, typically with a thickness less than or equal to 1.2 cm.1 We have been using the Manchester System for the past 3 decades, although sometimes external radiation was also combined with cesium (Cs) brachytherapy. Hosokawa, et al.2 has reported on the treatment results of combination therapy at our institute. The percent of patients with 5 year local control for T1 and T2 diseases were 92.6% and 62.7%, respectively.2 The value for T1 disease was comparable with the results achieved at other institutions. However, the T2 control rate was far from satisfactory, and therefore, in 1993, we changed the treatment protocol from combination therapy to brachytherapy alone (7000 cGy). Since then, the 3 year local control rates obtained for T2 disease was 88% (unpublished data). Compared to T1 disease, T2 disease covers a wide range of diseases; the horizontal and vertical lengths vary from 2 to 4 cm, and thickness is sometimes ambiguous. Among these characteristics, tumor thickness is the major concern when treatment policy is discussed at the head-and-neck tumor board, which consists of head-and-neck surgeons, oral surgeons, dentists, interventional radiologists, and radiation oncologists. The tumor board has met weekly since the 1970s. Every new patient with head-and-neck cancer has been examined at the same institution by doctors with different expertise. After discussion, possible treatment options are presented to patients and they choose their preferred treatment. When tumor thickness approaches 1.5 cm, our treatment options can vary considerably. From the perspective of a radiation oncologists, a two plane implant is required,1 although there is the high risk of necrosis of the mucosa and mandible. The University of Florida group reported that the incidence of severe complications were 9%.3 Surgeons hesitate in recommending a near hemiglossectomy particularly for young patients. This is because the procedures are to some extent associated with orocutaneous fistula, flap necrosis, dysphagia, and speech disturbance.2 Hence, treatments for tumors such as ours are controversial. In this short report, we discuss the difficulties regarding management of thick cancer of the tongue.
From August 2003 to February 2005, 5 patients with tumor thickness close to 1.5 cm were referred to our tumor board. Tumor thickness was evaluated manually and by magnetic resonance imaging. The well known Manchester System specifies using a single plane implantation only for tumors with a thickness of less than 12 mm. If the thickness exceeds 12 mm, a two plane implantation should be implemented. Fig. 1 shows the concept of a two plane implant. As shown, full strength Cs needles are implanted at the outer edge of the tongue. It is likely that the risk of mucosal necrosis will be high. We therefore modified this implant system. According to this modification, the mucosal surface will still receive 7,000 cGy, but within the tumor there will be 1.1-1.2 times higher dose points. Fig. 2 is a representation of our modified implant. This is an ideal implantation. In reality, the outer needles were inserted up to a depth of 2-3 mm from the mucosal surface. To reduce the dose to the mandible, we used a custom-made spacer for each patient as described previously.4 Cs implantation was carried out by T.N., and it was performed under general anesthesia in all cases. The needles' position was observed by a portable diagnostic X-ray machine, and if needed, reinsertion of a needle was performed. A nasopharyngeal airway was kept in place for a few days to prevent possible air way obstruction due to edema. Patients were examined using computed tomography (CT) to confirm good 3-dimentional needle alignment. The image was also used to measure the horizontal length of the needles for dose calculation. In both planes, the needles were inserted according to the Manchester System. The dose was prescribed at the plane 0.5 cm away from the source plane (coincidentally, this value meant that the prescribed dose was on the mucosal surface, as the tumor thickness was about 1.5 cm in all patients). The dose rate at the mucosal surface varied from 1,211 cGy/day to 1,410 cG/day with a mean dose of 1,262 cGy/day. The mean dose of the inner plane was 1,469 cG/day with a range from 1,392 cGy/day to 1,499 cGy/day. In one patient, a needle implanted near the base of the tongue was difficult to remove, and the needle was removed under general anesthesia.
Table 1 shows the characteristics and outcomes of the patients. It also includes the time to be spent for insertion. One patient died from metastasis to the lung, and another patient died from metastasis to the lymphnodes. The former patient (Pt. 3) needed 2.5 hours for insertion (i.e., reinsertion was necessary to obtain an ideal configuration of needles). The patient with lymph node metastasis (Pt. 1) refused selective neck dissection because for cosmetic reasons, and developed repeated nodal metastases. Node removal was performed whenever metastasis developed. In all the patients who developed nodal recurrences, their primaries were preserved. Acute mucositis (RTOG Grade 3) subsided within one month after removal of needles in all patients and there have been no mucosal bleedings. At 6 months, all patients developed atrophy (RTOG Grade 2) of the tongue. However this did not cause any inconvenience to the patients.
Fig. 3 shows a typical case with a 1.5 cm-thick T2 tumor. The oral surgeons' recommendation was a near-hemiglossectomy. In addition, they believed that even if brachytherapy succeeded, because of the risk of nodal relapse the primary and the nodal area should be removed en bloc. This is because they think that if a primary recurs after a neck dissection, it will be almost impossible to salvage the patient. The head-and-neck surgeons had an intermediate opinion. They thought that the tumor was curable with either an operation or brachytherapy, although surgery would provide higher local control. Because of the patient's age, and the resulting undesirable cosmetic effect, disturbance in speech, swallowing, and taste, secondary to surgical intervention, brachytherapy was recommended. Radiation oncologists were a little hesitant in performing brachytherapy because of the high complication rate; however, the patient decided to be treated with brachytherapy. To minimize complications, we performed Cs implantation with the modified Manchester System mentioned above. At 2.5 years after the treatment, the patient has had no signs of complications or loco-regional recurrence.
It is well known that for carcinoma of the tongue, tumor thickness is one of the prognostic factors of regional nodal recurrence.5,6 Our nodal recurrence rate was 60% (3/5), which is consistent with those reports. It should be noted that there has been no nodal recurrence among the 3 patients treated with selective neck dissection. There have been no reports on the relationship between Cs insertion time and outcome, but there might have been tumor cell seeding during the prolonged procedure (Pt. 3). Selective neck dissection has gradually gained popularity, and several studies have reported its efficacy.7-10 It is unlikely that our patients will develop further recurrence in the neck, as around 90% of lymphnode relapse occurs within 1 year of the first event.12 There has been controversy regarding how to manage a clinically controlled tongue in a patient who develops nodal disease. In our institute, although there is no definitive policy, a large primary that was initially thought to be uncontrollable by brachytherapy is a candidate for neck dissection with near-hemiglossectomy. The Kyushu University Group analyzed 396 patients who were treated with brachytherapy and concluded that glossectomy is unnecessary if primary sites were clinically controlled.11 However, the number of cases with nodal dissection with glossectomy was only 6 and their T stage was not documented.
In the present study, we used magnetic resonance imaging (MRI) to measure tumor thickness. The tumor border was clearly seen in all cases. This is very helpful in making a treatment decision and also for simulating Cs implantation. The inner tumor border is sometimes ambiguous. Our current diagnostic procedures include MRI, CT, and positron emission tomography (PET). The high local control rate in this study might have been achieved because of the careful treatment planning based on these images. As to the local control rate, Hareyama, et al.13 reported treatment results of 130 patients treated with brachytherapy. Their local control rate for T3 cases was 70.9%.13 Since our cases were nearly T3 in terms of tumor thickness, in the sense that they needed two-plane implantation, our treatment results are comparable with theirs. The aforementioned study indicates that the overall incidence of ulceration of the tongue and mandibular complication was 20% (26/130) and 13% (17/130), respectively.13 Although the follow-up period is still short, our patients did not show any signs of complications in soft tissues and mandibles. We would like to believe that this was due to our modified method of insertion. A multidiscipline approach is also important in deciding on a treatment plan. Previously, we reported a T4 tongue cancer that was treated with intra-arterial chemotherapy and brachytherapy using high-dose-rate (HDR) iridium.14 The patient continues to be free from diseases and complications 7 years after treatment. To the best of our knowledge, patients being evaluated at the same time by physicians with different expertise, followed by a discussion on the best treatment plan, are rare in Japan and abroad. In many institutions, patients are simply "referred" to the radiation oncology department. The multidisciplinary approach is an established tradition in our university and it is what we want to continue in the future.
Dose heterogeneity occurs with the modified Manchester System; in other words, the outer surface receives 7,000 cGy but the inner 0.5-cm plane may receive 8,000 cGy. We initially thought that a few patients would develop a persistent mucosal ulcer. However, to our surprise, no patients developed any signs of a mucosal ulcer. This could indicate that the presence of the high dose area in deep muscle (far from the mucosa surface) does not lead to a mucosal ulcer. The T4 case mentioned above also received 30 Gy external radiation and 48 Gy in 12 HDR fractions, and did not develop any signs of a mucosal ulcer. Although the number of cases is too small to discover the reason, good needle arrangement may be a factor in both local control and the absence of complications. For the past 15 years, most brachytherapy, including all 5 cases in the present report, were performed by T.N. After a discussion between the authors, H.S, and M.N. regarding the reason for the low primary control rates in T2 cancer, M.N. suggested that external radiation should be omitted, and in 1993 we changed the treatment policy to 7,000 cGy using Cs implants only. A learning curve may have also contributed to the good local control in the present series. This is not based on science, but the success of brachytherapy seems to depend on a physician's enthusiasm. Cs implants for tongue cancer are no longer available at a hospital in Manchester since the specialist for this treatment modality retired (personal communication).
In summary, although the number of patients in this series was small, the modified Manchester System resulted in good local control and no complications. Evaluation of tumor geometry by advanced diagnostic tools is important for treatment planning, and good needle alignment is essential. Increasing the number of patients treated by this technique is required to make a definitive conclusion.
Figures and Tables
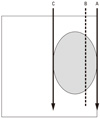 | Fig. 1Original Manchester System for T2N0 with a thickness of 1.5 cm. The tumor is shown sandwiched between full length needles. Dose is prescribed at the 0.5 cm plane B from the lateral border of the tongue. Overdose is a concern at plane A. |
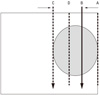 | Fig. 2Modified Manchester System at Hokkaido University. Insert full intensity needles 5 mm from the lateral border of the tongue. Half intensity needles were implanted at the medial border of the tumor. Dose is prescribed at plane A. Dose at plane D, which is 1.1 - 1.2 time higher than that at the plane A, is a concern. |
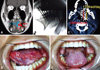 | Fig. 3(A) Gadolinium-enhanced MR image clearly revealed the tumor. The thickness was 15.3 mm and the height was 27.5 mm. (B) Operating room X-ray image. A total of 15 Cs needles were implanted. Their alignment was satisfactory. (C) CT image immediately after implant. The lateral needles were implanted 0.5 cm from the mucosal surface. The inner needles were placed 1.0 cm from the lateral needles. This alignment was the same as in the simulation. The dose prescription plane is highlighted in yellow (1,198 rad/day) and the inner plane 5 mm away from the outer needle plane is highlighted in red (1,437 rad/day). The dose at the red plane will end up with 20% higher than that of the lateral portion of the tongue. (D) Appearance of the tongue mucosa 2.5 years after the implant. The mucosa appears slightly white, there is no sign of tissue necrosis. (E) Slight atrophy is seen in the area of the implant. When the tongue protrudes, a slight deviation to the left is observed. |
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid for Scientific Research (B20390319), which was provided by the Ministry of Education, Science and Culture of Japan.
References
1. Duthie MB, Gupta NK, Pointon CS. Pointon RCS, editor. Head and neck. The radiotherapy of malignant disease. 1991. London: Springer-Verlag;156.

2. Hosokawa Y, Shirato H, Nishioka T, Tsuchiya K, Chang TC, Kagei K, et al. Effect of treatment time on outcome of radiotherapy for oral tongue carcinoma. Int J Radiat Oncol Biol Phys. 2003. 57:71–78.

3. Mendenhall WM, Riggs CE, Cassisi NJ. Devita VT, Hellman S, Rosenberg S, editors. Treatment of head and neck cancers. Cancer principle and practice of oncology. 2005. Philadelphia: Lippincott Williams and Wilkins;682.
4. Obinata K, Ohmori K, Tuchiya K, Nishioka T, Shirato H, Nakamura M. Clinical study of a spacer to help prevent osteoradionecrosis resulting from brachytherapy for tongue cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003. 95:246–250.

5. O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer--but what thickness? Head Neck. 2003. 25:937–945.
6. Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma-A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002. 24:513–520.

7. Mukhija V, Gupta S, Jacobson AS, Anderson Eloy J, Genden EM. Selective neck dissection following adjuvant therapy for advanced head and neck cancer. Head Neck. 2009. 31:183–188.

8. Frank DK, Hu KS, Culliney BE, Persky MS, Nussbaum M, Schantz SP, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope. 2005. 115:1015–1020.

9. van der Putten L, van den Broek GB, de Bree R, van den Brekel MW, Balm AJ, Hoebers FJ, et al. Effectiveness of salvage selective and modified radical neck dissection for regional pathologic lymphadenopathy after chemoradiation. Head Neck. 2009. In press.

10. van der Putten L, van den Broek GB, de Bree R, van den Brekel MW, Balm AJ, Hoebers FJ, et al. Effectiveness of salvage selective and modified radical neck dissection for regional pathologic lymphadenopathy after chemoradiation. Head Neck. 2009. 31:593–603.

11. Nishio M, Sakurai T, Kagami Y, Narimatsu N, Saito A, Hareyam M, et al. [Results of brachytherapy of tongue cancer]. Gan No Rinsho. 1986. 32:339–344.
12. Urashima Y, Nakamura K, Kunitake N, Shioyama Y, Sasaki T, Ooga S, et al. Is glossectomy necessary for late nodal metastases without clinical local recurrence after initial brachytherapy for N0 tongue cancer? A retrospective experience in 111 patients who received salvage therapy for cervical failure. Jpn J Clin Oncol. 2006. 36:3–6.
13. Hareyama M, Nishio M, Saito Y, Kagami Y, Asano K, Oouchi A, Narimatsu N, et al. Results of cesium needle interstitial implantation for carcinoma of the oral tongue. Int J Radiat Oncol Biol Phys. 1993. 25:29–34.

14. Nishioka T, Homma A, Furuta Y, Aoyama H, Suzuki F, Ohmori K, et al. A novel approach to advanced carcinoma of the tongue: cases successfully treated with combination of superselective intra-arterial chemotherapy and external/high-dose-rate interstitial radiotherapy. Jpn J Clin Oncol. 2006. 36:822–826.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download