Abstract
Purpose
Atherosclerosis is characterized by the progressive deposition of lipids and inflammatory process. We attempted to develop a chemically-induced hyperlipidemic mice model, using poloxamer-407 and evaluated the lipid lowering and anti-inflammatory effect of P. notoginseng compared with that of atorvastatin, an antihyperlipidemic drug.
Materials and Methods
Male Wistar rats were randomly divided into 5 groups: control group without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100). After 3 weeks, we measured serum total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride, interleukin (IL)-1, tumor necrosis factor (TNF)-α levels, and reports of cyclo-oxygenase (COX)-2 & intercellular adhesion molecule (ICAM) appearances in each group.
Results
After 3 weeks, serum cholesterol levels significantly decreased in P + ST and P + NG40 groups. Significant decrease of LDL level was only noted in the P + ST group. P + ST, P + NG40, and P + NG100 also had decreased serum triglyceride levels; however, P + ST and P + NG40 showed no statistical difference of the triglyceride lowering effect. The results of IL-1 and TNF-α and the appearance of COX-2 and ICAM were statistically not different in each group.
Blood lipid consists of cholesterol, triglyceride, phospholipid, and free fatty acid (FFA). These flow through the blood vessels in the form of lipoprotein. Hyperlipidemia designates the case where lipids are not normally transported due to the problems in lipoprotein synthesis and analysis. Hyperlipidemia is a key factor of arteriosclerosis, a pathophysiologic factor of vascular diseases such as angina pectoris, myocardial infarction, and stroke. Thus, it is very important to properly maintain the blood-lipid concentration in the prevention and early treatment of hyperlipidemia.1,2
In light of traditional Korean medicine, hyperlipidemia designates the case where blood becomes smeary and clotted, which is related with obesity, circulatory disorders, metabolic disorders, intracorporal clamminess, intracorporal heat, circulatory congestion and otherwise defined by traditional Korean medicine. Also, symptoms such as bacterial hematic fever, bloody sweat, anemic condition, and exhaustion of vital energy are regarded as the etiology. To be brief, in light of traditional Korean medicine, hyperlipidemia is closely related with the disharmony among the spleen, the heart, the liver, and the kidney. From the viewpoint of modern medical science, hyperlipidemia indicates when blood-lipid concentration is in the top 10%. Such a symptom can be explained under the category of abnormal body wastes and blood congestion in traditional Korean medicine.3
Sam-Chil-Geun [Panax notoginseng (Burk) F. H. Chen] is similar to ginseng. It is not indigenous to Korea, but to China.4 It has been traditionally used as both internal medicine and also external preparation against blood congestion and extravasation. Also, it helps circulate blood, decongest blood circulation, treat inflammations, and relieve pain. Moreover, it has been recently reported that Sam-Chil-Geun is effective against the angina pectoris caused by coronary arteriosclerosis. Since Sam-Chil-Geun is effective in decongesting blood circulation, the above result might be possible.5 This suggests that hyperlipidemia may be treated in traditional Korean medicine, along with Sam-Chil-Geun.
Recently, Park and Wang6 reported that the mixture of the musk, the ox bezoar, and Sam-Chil-Geun significantly lowered the total cholesterol, phospholipids, and low-density lipoprotein (LDL)-cholesterol of rabbits with hyperlipidemia induced by high-cholesterol diets. Likewise, Kim7 reported that the Sam-Chil-Geun extract significantly lowered the total cholesterol, LDL-cholesterol, and triglyceride of the rats with hyperlipidemia induced by high-cholesterol diets. In addition, it was reported that anti-inflammatory treatment may be effective against hyperlipidemia because hyperlipidemia is closely related to the C-reactive protein and tumor necrosis factor (TNF)-α.8 According to a recent study, inflammatory response is directly connected with the initial symptom of arteriosclerosis, not to mention hyperlipidemia.9 As such, hyperlipidemia and inflammatory responses need to be understood cooperatively. In this context, the results of this study deserve attention.
This research therefore involved rats induced with hyperlipidemia through the administration of poloxamer-407 to examine the change in lipid level in blood for each Panax notoginseng administration level compared with atorvastatin administration. In addition, the interleukin (IL)-1, TNF-α, cyclo-oxygenase (COX)-2, and intercellular adhesion molecule (ICAM) manifestation level was examined through immunohistochemistry staining of the abdominal aorta, drawing significant results for the report.
Six-week-old male Wister rats (Orient Co., Seoul, Korea) were used as experimental animals. They weighed 140 ± 10 g on average. Experimental animals adapted themselves to the temperature of 22℃ ± 2 and the relative humidity of 60 ± 10% for one week. Light was alternated with darkness at intervals of 12 hours (07:00 a.m-07:00 p.m). Water and sold feed (Samyang Feed Co., Pyeongtaek, Korea) were unlimitedly supplied to the rats during the experiment.
Reflux extraction was applied to a round-bottom flask containing 1 kg of Sam-Chil-Geun and 5 liters of distilled water for 2 hours. The filtrate collected through a 100-mesh strainer was concentrated at 60℃ by a decompression concentrator (Eyela NE, Tokyo Rikakikai Co. Ltd., Tokyo, Japan). A ropy liquid was thus produced. The liquid was freeze dried (PVTFD 10A, Ilshin Lab. Co. Ltd., Suwon, Korea) and so 118 g of Sam-Chil-Geun extract was produced (the yield of 11.8%). To the positive control group, 1.34 mg/kg of Atorvastatin (Lipitor; Pfizer Inc., Seoul, Korea) was administered.
Experimental animals were divided into the normal control group (the normal group), the control group from which hyperlipidemia was induced by using of poloxamer-407 (P group), the positive control group to which poloxamer-407 and atorvastatin were administered (P + ST group), the first experimental group to which poloxamer-407 and 40 mg/kg of Sam-Chil-Geun extract were administered (P + NG40 group), and the second experimental group to which poloxamer-407 and 100 mg/kg of Sam-Chil-Geun extract were administered (P + NG100 group).
To the normal group, feed and water were unlimitedly supplied. To the other groups, poloxamer-407 was administered at 3 day intervals, from the first day of the experiment until the 21st day. Prior to the administration, poloxamer-407 was dissolved in saline and was kept refrigerated for one day. It was injected into the abdominal cavity at a dose of 500 mg/kg, between 10:00 a.m and 11:00 a.m. The experimental animals fasted for 6 hours after the injection.
To the P + ST group, Atorvastatin (Lipitor, Pfizer Inc., Seoul, Korea) was orally administered at a dose of 1.34 mg/kg, every day. To the P + NG40 group and the P + NG100 group, the Sam-Chil-Geun extract (the yield of 11.8%) was orally administered at a dose of 40 mg/kg and 100 mg/kg, respectively, during the same period. The extract was refrigerated and was defrosted at a concentration of 100 mg/mL prior to the administration by using Dimethyl Sulfoxide (DMSO). Also, the extract was administered 2 hours after polaxamer-407 was administered.
On the 21st day of the experiment, the ether-inhalation anesthesia was performed on all experimental animals that fasted for 12 hours. The etherized animals were again treated with 75% alcohol, and an abdominal incision was performed to take blood samples from the ascending aortas. The blood samples, kept at room temperature for 30 minutes, were centrifuged at a speed of 3,000 revolutions per minute (RPM) for 15 minutes. The serum, extracted from the centrifuged samples, was used to analyze the blood-lipid content.
To every group, total cholesterol, triglyceride, high-density lipoprotein (HDL)-cholesterol, and LDL-cholesterol were measured on the basis of the plasma-lipid profile.
A mixture of 0.02-mililiter serum and 3-mililiter enzyme solution was heated in a double boiler at 37℃ for 5 minutes. The optical absorbance was measured at a 500-nanometer wavelength.
A mixture of 0.02-mililiter serum and 3-mililiter reagent was heated in a double boiler at 37℃ for 5 minutes. The optical absorbance was measured at a 546-nanometer wavelength.
A mixture of 1-mililiter serum and 0.1-mililiter precipitant, kept at room temperature for 5 minutes, was centrifuged for 10 minutes and the supernatant separated the mixture for as much as 0.02 mL. The mixture of 0.02-mililiter supernatant and 0.1-mililiter precipitant, kept at room temperature for 5 minutes, was centrifuged for 10 minutes. Again, a mixture of 0.02-mililiter supernatant and 3-mililiter enzyme solution was heated in a double boiler at 37℃ for 15 minutes. The optical absorbance was measured at a 500-nanometer wavelength.
Ficoll-Hypague centrifugation was applied to the blood samples diluted by the same quantity of Hank's Balanced Salt Solution (HBSS) for 30 minutes (400 g; 2,500 rpm, 5 mins; 4℃). 50 microliters of supernatant was kept at minus 70℃ prior to the measurement. IL-1 and TNF-α were measured by using a ELISA Kit (Bio Source International Inc., Carlsbad, CA, USA).
On the 21st day of the experiment, the ether-inhalation anesthesia was performed on all experimental animals that fasted for 12 hours. On the etherized animals treated with 75% alcohol, an abdominal incision was performed. The tissue samples (a thickness of 4 micrometers) were obtained from the ascending aorta to the junction of the left renal artery. After paraffin was removed by the Bond Dewax Solution (Vision Bio System, Suwon, Korea), the tissue section was treated with the Bond ER Solution (Vision Bio System) at 100℃ for 30 minutes. It was again treated with hydrogen peroxide for 5 minutes and then reacted with the COX-2 monoclonal antibody (1 : 100, Dako Cytomation, Glostrup, Denmark) and the ICAM-1 monoclonal antibody (1 : 100, Novacastra Laboratories, Peterborough, UK) for 15 minutes. The biotin-free polymeric horseradish peroxidase linker antibody conjugate system of the Bond-Maxautomatic Slide Strainer (Vision Bio System) was used in this procedure. Subsequently, it was counterstained with hematoxylin with glycerin sealed and observed under optical microscopy.
The normal group, P group, P + ST group, P + NG40 group, and P + NG100 group measured an intra-serum total cholesterol level of 85.7 ± 4.9 mg/dL, 470.2 ± 52.0 mg/dL, 320.2 ± 77.6 mg/dL, 400.3 ± 64.2 mg/dL, and 451.1 ± 56.1 mg/dL, respectively.
The total cholesterol was significantly lowered in the P + ST group and the P + NG40 group, as compared with the P group. The results of the intergroup comparison indicate that the total cholesterol of the P + ST group was significantly lowered compared to P + NG40 (p < 0.05 in ANOVA and post-hoc) (Fig. 1).
The normal group, P group, P + ST group, P + NG40 group and P + NG100 group measured an intra-serum HDL-cholesterol level of 25.2 ± 2.6 mg/dL, 38.6 ± 7.3 mg/dL, 40.6 ± 7.3 mg/dL, 37.4 ± 6.3 mg/dL, and 35.2 ± 2.1 mg/dL, respectively. The intergroup difference was not significant, as compared to the P group (p < 0.05 in ANOVA) (Fig. 2).
The normal group, P group, P + ST group, P + NG40 group and P + NG100 group measured an intra-serum LDL-cholesterol level of 13.7 ± 5.8 mg/dL, 126.5 ± 26.9 mg/dL, 105.0 ± 17.6 mg/dL, 119.2 ± 11.5 mg/dL, and 132.3 ± 17.8 mg/dL, respectively.
The LDL-cholesterol was significantly lowered in the P + ST group compared to the P group (p < 0.05 in ANOVA and post-hoc) (Fig. 3).
The the normal group, P group, P + ST group, P + NG40 group and P + NG100 group measured an intra-serum triglyceride level of 51.5 ± 10.6 mg/dL, 1034.5 ± 141.2 mg/dL, 710.8 ± 78.1 mg/dL, 742.8 ± 84.2 mg/dL, and 853.0 ± 104.2 mg/dL, respectively.
Triglyceride was significantly lowered in the P + ST group and P + NG40 group compared to the P group. The results of the intergroup comparison indicate that the triglyceride of the P + ST group was significantly lowered compared to the P + NG100 group (p < 0.05 in ANOVA and post-hoc) (Fig. 4).
The intergroup difference was not observed in IL-1 and TNF-α (p < 0.05 in ANOVA) (Fig. 5).
Hyperlipidemia is largely distinguished into 'primary' and 'secondary' hyperlipidemia depending on its etiology. It was reported that primary hyperlipidemia develops when lipoprotein lipase (a type of the enzyme related to lipid metabolism) is deficient, when cell-membrane LDL receptors are insufficient, when apoprotein CII (a type of lipoprotein) is deficient, or when apoprotein E is abnormally produced. Secondary hyperlipidemia develops in relation to hormonal disorders such as diabetes or hypothyroidism, alcoholism, the abuse of glucocorticoids, and other diseases of the internal organs that may affect lipid metabolism (nephrotic syndrome, hepatoma, SLE, etc).
Blood lipid consists of cholesterol, triglyceride, phospholipid, and FFA absorbed from food or synthesized in vivo. The hydrophobic lipid, bound to hydrophilic apoprotein, flows through the blood vessels, in the form of lipoprotein. Lipoprotein is separated into chylomicron, very low-density lipoprotein (VLDL), LDL, and HDL according to the component ratios of triglyceride, cholesterol, and others. The specific gravity of lipoprotein increases in proportion to cholesterol but in inverse proportion to triglyceride or protein. VLDL contains a large amount of triglyceride, and LDL and HDL contains a large amount of cholesterol. If problems arise in lipoprotein synthesis or analysis, blood lipids are not normally transported and so its concentration becomes out of normal range.
Hyperlipidemia is one of the key factors that cause atherosclerosis in the coronary artery or cerebral blood vessels, in addition to hypertension, smoking, and obesity.8-10 The LDL, which was not removed in the process of lipid metabolism, is apt to flow into the subendothelial space, as well as to undergo oxidation. The oxidized LDL is phagocytized by the scavengers of macrophages. This fat-laden macrophage is left with the lipid core filled with cholesterol after necrocytosis and then makes arteriosclerosis.11 This is the main pathophysiologic factor of vascular diseases such as angina pectoris, myocardial infarction, stroke, etc. Thus, it is very important to properly maintain blood-lipid concentration in the prevention and early treatment of hyperlipidemia. In particular, LDL-cholesterol and triglyceride needs to be intensely managed because the increase of either may bring forth more serious danger. However, the decrease of HDL-cholesterol may also be dangerous because it takes part in the removal of the cholesterol left in cellular tissue.
It was already proven that Sam-Chil-Geun is effective in preventing platelet aggregation, that it increasesthe blood flow rate of the coronary artery, it helps the left ventricle to relax, and it reduces the risk of heart diseases.12 Also, it was reported that Sam-Chil-Geun lowers the concentration of intra-neutrophil free calcium so that inflammations may be healed.13 The notoginsenosides of Sam-Chil-Geun seemed to stimulate tissue-type plasminogen activators in human endotheliocytes and vascular smooth muscle cells cultured in the medium, and to deactivate plasminogen activator inhibitor-1.14 Prostacyclin synthesis was promoted in the coronary artery of the rats with Sam-Chil-Geun extract orally administered for 10 days, and thromboxane A2 synthesis was deactivated with decrease of arteriosclerotic plaque.15
Arteriosclerosis is a response not only to hyperlipidemia but also to the inflammations of the vessel wall. Such inflammatory responses can be explained through the hypofunction of vascular endotheliocytes, the development of adhesion molecules, and the infiltration of neutrophils, that is, the monocytes, which infiltrated the arterial intima and rapidly changed to macrophages.16-18 NF-kB, cAMP response element-binding protein (CREB) and activating protein (AP)-1, which are known as nuclear transcription factors, call forth pro-inflammatory factors such as COX-2 and TNF-α and IL-1. On the other hand, it is known that Sam-Chil-Geun inhibits such factors from developing.19 Another study reported that Sam-Chil-Geun inhibits inflammation-inducing substances such as COX-2, iNOS, and cell adhesion molecules by the NF-kB pathway.20 Sam-Chil-Geun comprehensively consists of about 20 types of saponin such as ginsenoside Rg1, Rg2, Rb1, Rb2, Rb3, Rc, Rd, Re, Rh, F2, and notoginsenosides R1, R2, R3, R4, R6, Fa, Fc, and Fe.21 However, the mechanism how these saponins and ginsenoside interact and make effect on lipid metabolism and inflammatory change has not yet to be clarified.
As in previous studies,6-8 hyperlipidemia was artificially induced from experimental animals by feeding these animals a high-cholesterol diet. But in this study, Polyxamer-407 was administered to make hyperlipidemia and arteriosclerosis instead of fat diet. Polyxamer-407 is a block copolymer in which the polyoxyethylene unit is alternated with a polyoxypropylene unit,22 and it is known that the reagent increases neutral fat and cholesterol by at least 60 times and 8 times, respectively.23 Lipoprotein lipase is necessary to hydrolyze the lipoprotein TG of serum, and an in vitro experiment found that Polyxamer-407 inhibits lipoprotein lipase. Recent study showed that the activity of lipoprotein lipase decreased by 95% in the group to which Polyxamer-407 was administered for 3 hours.24 In the process of cholesterol synthesis, hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase functions as the main rate-limiting enzyme. Lovastatin and pravastatin inhibited cholesterol synthesis by acting as HMG-CoA reductase inhibitors.25
In the present study, the Sam-Chil-Geun extract was administered to the rats with hyperlipidemia induced by the administration of poloxamer-407 in order to ascertain whether blood-lipid, IL-1, TNF-α, COX-2, and ICAM can be lowered. In particular, COX-2 and ICAM were immunohistochemically stained in the process of the experiment. To the positive control group, Atorvastatin (one of HMG-CoA reductase inhibitor) was used.
With regards to total cholesterol, the P + ST group and P + NG40 group showed a significant decrease compared to the control group from which hyperlipidemia was artificially induced (P group). In the results of the intergroup comparison, the total cholesterol level of the P + ST group was significantly lowered compared to the P + NG40 group. In the case of HDL-cholesterol, the intergroup difference was not significant as compared with the control group from which hyperlipidemia was artificially induced (P group). Only the P + ST group showed a significant decrease of LDL cholesterol level. Triglyceride was significantly lowered in the P + ST group and P + NG40 group compared to the control group from which hyperlipidemia was artificially induced (P group). In the results of the intergroup comparison, the P + ST group showed a significantly lower triglyceride level than the P + NG100 group. But there was no significant difference of triglyceride level between the P+ST group and the P + NG40 group.
Overall, it was found that Sam-Chil-Geun lowers the total cholesterol level and the triglyceride level. Since there was no significant difference among the 100 mg/kg, 40 mg/kg of the Sam-Chil-Geun extract and Atorvastatin in the decrease of triglyceride, there is a possibility that 40 mg/kg may be more effective than 100 mg/kg in the decrease of lipids. The Sam-Chil-Geun needs to be further researched with subgrouping by its concentration. And we also suggest the study of combination treatment of the Sam-Chil-Geun extract and Atorvastatin to maximize the curative effect of hyperlipidemia and to minimize their doses according to patient's symptoms and disease severity in the near future.
Figures and Tables
 | Fig. 1Serum total cholesterol levels in each group. Without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (*p < 0.05, ANOVA & post-hoc). ANOVA, analysis of variance. |
 | Fig. 2Serum HDL-cholesterol levels in each group. Without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (p < 0.05, ANOVA & post-hoc). ANOVA, analysis of variance. |
 | Fig. 3Serum LDL-cholesterol levels in each group. Without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (*p < 0.05, ANOVA & post-hoc). ANOVA, analysis of variance. |
 | Fig. 4Serum triglyceride levels in each group. Without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (*p < 0.05, ANOVA & post-hoc). ANOVA, analysis of variance. |
 | Fig. 5IL-1 (A), TNF-α (B) levels in each group. Without any intervention (Normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (p< 0.05, ANOVA & post-hoc). IL-1, interleukin-1; TNF, tumor necrosis factor; ANOVA, analysis of variance. |
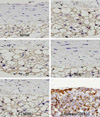 | Fig. 6Immunohistochemical COX-2 stained slides in each group (×400). Without any intervention (normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (p < 0.05, ANOVA & post-hoc). COX-2, cyclo-oxygenase-2; ANOVA, analysis of
variance. |
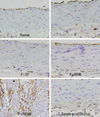 | Fig. 7Immunohistochemical ICAM stained slides in each group (×400). Without any intervention (Normal), poloxamer 500 mg/kg i.p. (P), poloxamer plus atorvastatin 1.34 mg/kg p.o. (P + ST), poloxamer plus P. notoginseng 40 mg/kg p.o. (P + NG40), and poloxamer plus P. notoginseng 100 mg/kg p.o. (P + NG100) (p < 0.05, ANOVA & post-hoc). ICAM, intercellular adhesion laboratories; ANOVA, analysis of variance. |
References
1. Teng CM, Kuo SC, Ko FN, Lee JC, Lee LG, Chen SC, et al. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim Biophys Acta. 1989. 990:315–320.

2. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993. 362:801–809.

3. Chang GT, Kang SK, Kim JH, Chung KH, Chang YC, Kim CH. Inhibitory effect of Korean herbal medicine, Dae-Jo-Whan, on platelet activating factor-induced platelet aggeregation. J Ethnopharmacol. 2005. 102:430–439.

4. Kuo SC, Teng CM, Lee JC, Ko FN, Chen SC, Wu TS. Antiplatelet components in Panax ginseng. Planta Med. 1990. 56:164–167.
5. Wang G, Wang L, Xiong ZY, Mao B, Li TQ. Compound salvia pellet, a traditional Chinese medicine, for the treatment of chronic stable angina pectoris compared with nitrates: a meta-analysis. Med Sci Monit. 2006. 12:SR1–SR7.
6. Jin UH, Park SG, Suh SJ, Kim JK, Kim DS, Moon SK, et al. Inhibitory effect of Panax notoginseng on nitric oxide synthesase, cyclo-oxtgenase-2 and neutrophil functions. Phytother Res. 2007. 21:142–148.

7. Wang J, Xu J, Zhong JB. Effect of Radix notoginseng saponins on platelet activating molecule expression and aggregation in patients with blood hyperviscosity syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004. 24:312–316.
8. Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hypertlipidemic individuals. Atherosclerosis. 2009. 204:476–482.
9. Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008. 79:1544–1551.

10. Wout ZG, Pec EA, Maggiore JA, Williams RH, Palicharla P, Johnston TP. Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injection to rats. J Parenter Sci Technol. 1992. 46:192–200.
11. Beckmann N, Cannet C, Babib AL, Bié FX, Zurbruegg S, Kneuer R, et al. In vivo visualization of macrophage infiltration and activity in inflammation using magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009. 1:272–298.
12. Cicero AF, Bandieri E, Arletti R. Orally administered Panax notoginseng influence on rat spontaneous behaviour. J Ethnopharmacol. 2000. 73:387–391.

13. Liu S, Chen JX. [Anti-arrhythmic effect of total saponins of Panax notoginseng]. Zhongguo Yao Li Xue Bao. 1984. 5:100–103.
14. Zhang WJ, Wojta J, Binder BR. Effect of notoginsenoside R1 on the synthesis of components of the fibrinolytic system in cultured smooth muscle cells of human pulmonary artery. Cell Mol Biol (Noisy-le-grand). 1997. 43:581–587.
15. Park HJ, Rhee MH, Park KM, Nam KY, Park KH. Effect of non-saponin fraction from Panax ginseng on cGMP and thrombocane A2 in human platelet aggregation. J Ethnopharmacol. 1995. 49:157–162.

16. Taubes G. Cardiovascular disease. Does inflammation cut to the heart of the matter? Science. 2002. 296:242–245.
17. Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002. 91:281–291.

19. Karin M. Inflammation-activated protein kinases as targets for drug development. Proc Am Thorac Soc. 2005. 2:386–390.

20. Abate A, Oberle S, Schröder H. Lipopolysaccharide-induced expression of cyclooxygenase-2 in mouse macrophages is inhibited by chloromethylketones and a direct inhibitor of NF-kappa B translocation. Prostaglandins Other Lipid Mediat. 1998. 56:277–290.

21. Liu Y, Xie MX, Kang J, Zheng D. Studies on the interaction of total saponins of Panax notoginseng and human albumin by Fourier transform infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2003. 59:2747–2758.
22. Johnston TP, Punjabi MA, Froelich CJ. Sustained delivery of interleukin-2 form a poloxamer 407 gel matrix following intraperitoneal injection in mice. Pharm Res. 1992. 9:425–434.
23. Wout ZG, Pec EA, Maggiore JA, Williams RH, Palicharla P, Johnston TP. Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injections to rats. J Parenter Sci Technol. 1992. 46:192–200.
24. Johnston TP, Palmer WK. Mechanism of poloxamer 407-induced hypertriglyceridemia in rat. Biochem Pharmacol. 1993. 46:1037–1042.
25. Porter JA, Carter BL, Johnson TP, Palmer WK. Effects of pravastatin on plasma lipid concentrations in poloxamer 407-induced hyperlipidemic rats. Pharmacotherapy. 1995. 15:92–98.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download