Abstract
Purpose
The gender difference of neurally mediated syncope is not well defined in a large patient population. The aim of this study was to evaluate the gender difference of clinical manifestations in patients with neurally mediated syncope who underwent head-up tilt test.
Materials and Methods
The medical records of 1,051 consecutive patients with two or more episodes of syncope, who were diagnosed as having neurally mediated syncope by head-up tilt test, were retrospectively reviewed.
Results
Of 1,051 patients, 497 (47.3%) patients were male and 554 (52.7%) patients were female. Female patients were experiencing syncopal episodes for longer periods of their lives (8.2 ± 9.5 years vs. 6.8 ± 9.2 years, p = 0.002) and more episodes of syncope prior to head-up tilt test (HUT) (7.2 ± 9.4 vs. 5.0 ± 6.4, p = 0.001) than male patients. Micturition syncope (20.0% vs. 5.2%, p < 0.001) was observed more frequently in male patients than in female patients. To the contrary, however, defecation syncope (16.3% vs. 9.3%, p < 0.001) was observed more frequently in female patients than in male patients.
Syncope is a frequently observed symptom, and the prevalence in the general population is 3.0 percent in men and 3.5 percent in women.1-4 Neurally mediated syncope is the most common cause of syncope, which account for 28.7 percent in all episodes of syncope.1 It often occurs in response to orthostatic stimulus (prolonged standing), other nonorthostatic stimuli (fear, emotional stress, pain), and a variety of activities (micturition, defecation, coughing, swallowing, postprandial, and pressure on the carotid sinus). These stimuli transiently cause a sudden failure of the autonomic nervous system resulting in hypotension and bradycardia, which eventually cause syncope.5 Detailed history and physical examination are both central to the diagnosis of neurally mediated syncope. Head-up tilt test (HUT) is used to confirm the diagnosis of neurally mediated syncope in suspected case.6,7 Previous studies reported the clinical features of neurally mediated syncope.8-10 Neurally mediated syncope is widely believed to be more common in females than in males.11 However, the gender difference of recurrent neurally mediated syncope has not well been evaluated in a large patient population.
The objective of this study was to evaluate the gender difference of clinical manifestations in patients with recurrent neurally mediated syncope who underwent HUT at a single tertiary referral center.
The patient populations were drawn from HUT data base at Samsung Medical Center, Seoul, Korea between January 1995 and February 2006.
We defined the episode of syncope as a sudden and brief loss of consciousness with a loss of postural tone and spontaneous recovery from the loss of consciousness. We counted episodes of syncope from HUT data base at Samsung Medical Center.
Patients were included if they had had two or more episodes of syncope before HUT and showed a positive response of HUT. Patients were excluded from the study if they had other causes of syncope (including orthostatic syncope, neurologic cause, cardiac cause, unknown cause, and medication-related syncope). One thousand and fifty-one consecutive patients with neurally mediated syncope, diagnosed following HUT, were eligible for this study, and their records were retrospectively reviewed. The study was approved by the Regional Committee for Ethnics in Medical Research. We also defined patient's syncopal episode as specific situational syncope (micturition or defecation syncope) if he/she experienced syncopal episodes under more specific situation.
All patients underwent HUT in fasting state after obtaining informed consent. HUT consisted of two phases. The first phase of HUT was performed while patients were tilted to an angle of 70° for 30 minutes, or until symptoms appeared. If the first phase produced a negative response, the second phase with isoproterenol provocation was performed while maintaining the same degrees of tilting as the first phase for 15 minutes. Isoproterenol was intravenously administered at an initial rate of 1 µg/min. The infusion rate was increased by 1 µg/min every 3 minutes to a maximum of 5 µg/min. Electrocardiogram (ECG) was continuously monitored. Each patient's blood pressure was non-invasively measured beat-to-beat using a Finapres (OhMeda Monitoring System, Englewood, CO, USA) during the HUT.12 A positive response of HUT was defined when syncope or presyncope was reproduced in association with hypotension (the systolic blood pressure < 80 mmHg), bradycardia (a sinus arrest > 3 seconds or heart rate < 45 beats/min in the first phase, heart rate < 60 beats/min in the second phase), or both. A positive response of HUT was defined when syncope or presyncope was reproduced in association with hypotension. Positive responses were classified into three types (vasodepressive, cardioinhibitory, mixed) according to the criteria provided in the previous study: A vasodepressive response was defined as significant systolic blood pressure decrease < 80 mmHg. A cardioinhibitory response was defined as abrupt sinus arrest or heart rate decrease (sinus arrest > 3 seconds or heart rate < 45 beats/min in the first phase, heart rate < 60 beats/min in the second phase). A mixed response was defined as significant decrease of systolic blood pressure and heart rate.13
Means were calculated for continuous variables, and frequencies were measured for categorical variables. Distributions, which were not normally distributed, were described as medians and interquartile ranges. Comparisons were made by Wilcoxon rank-sum test for continuous variables, and the Chi-square test was used for categorical variables. A p value < 0.05 was considered statistically significant. Data were analyzed with statistical analysis system (SAS) software, version 9.13 (SAS Inc., Cary, NC, USA).
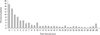
Of 1,051 patients, 497 (47.3%) patients were male and 554 (52.7%) patients were female. One hundred and thirty-six (13.0%) patients suffered defecation syncope and 128 patients (12.2%) had micturition syncope. The mean age of the study subjects was 36.0 ± 16.4 years at HUT. The first syncopal episode developed at ages 2-75 years in a skewed distribution, with a mode of 15 years, a median age of 22 years [interquartile ranges (IOR): 15, 38], and mean age of 27.8 ± 16.2 years. The mean time interval between first and last syncopal episodes was 7.5 ± 9.4 years. They had a mean 6.2 ± 15.5 syncopal episodes prior to HUT (Table 1). The first onset of neurally mediated syncope occurred most frequently at the age interval of 15-19 years old (Fig. 1). Although the recurrence of syncope decreased over time, 33 patients (3.18%) still experienced recurrent episodes 30 years after the first syncope (Fig. 2).
The patterns of a positive HUT were as follows. The vasodepressive type was noted in 726 (69.1%) patients, the cardioinhibitory type in 68 (6.5%) patients, and the mixed type in 257 (24.4%) patients (Table 1).
Ages at HUT and the first syncope were not significantly different between male and female patients. However, female patients had longer time interval between first and last syncopal episodes (8.2 ± 9.5 years vs. 6.8 ± 9.2 years, p = 0.002) and more episodes of syncope prior to HUT (7.2 ± 9.4 vs. 5.0 ± 6.4, p = 0.001) than male patients. Micturition syncope (20.0% vs. 5.2%) was observed more frequently in male patients than in female patients. To the contrary, however, defecation syncope (16.3% vs. 9.3%) was observed more frequently in female patients than in male patients (Table 2). The pattern of positive HUT was not significantly different between two groups (Table 3).
Our study showed that there were significant gender differences in the time interval of syncopal episodes, the frequency of syncope, and situational syncope. Female patients were experiencing syncopal episodes for longer periods of their lives (8.2 ± 9.5 years vs. 6.8 ± 9.2 years, p = 0.002) and more frequently experienced syncope (7.2 ± 9.4 vs. 5.0 ± 6.4 years, p = 0.001) than male patients. Soteriades, et al.1 evaluated the cause of syncope according to gender in the Framingham Heart Study from 1971 to 1998. However, they simply stated the percentage of vasovagal syncope and all situational syncopes according to gender, but did not evaluate the clinical characteristics of gender difference in both groups. Therefore, the present study is the first to show gender difference in the clinical manifestations of recurrent neurally mediated syncope in a large patient population.
This finding suggests that female patient may be prone to experience syncopal episodes more easily than male patients. Our finding was also supported by previous several studies,14-16 which showed gender differences in orthostatic tolerance and response to lower negative body pressure in healthy normal subjects. Women are more susceptible to orthostatic intolerance in warm environments. Orthostatic stress tends to be accentuated in the heat due to redistribution of central blood volume to the peripheral vascular bed. Fluctuations of the female sex hormones accompanying the menstrual cycle have been shown to have numerous effects on the physiological parameters.17 Recently, Meendering, et al.18 evaluated the influence of menstrual cycle and gender on the hemodynamic responses to combined orthostatic and heat stress, and found that men had greater orthostatic tolerance than women during combined upright tilting and passive heating.
In the present study, neurally mediated syncope occurred most commonly at the age interval of 15-19 years old. Sheldon, et al.8 also reported that the most common age of first faint was 13 years old, which was very similar to our result. However, we did not demonstrate any significant gender difference in the age of first syncope between male and female patients.
Situational syncope, such as micturion syncope or defecation syncope, is a subtype of neurally mediated syncope. In our study, a clear gender difference was found also in situational syncope. Micturition syncope is well known situational syncope, occurring at the end of urination or shortly thereafter. Micturition syncope (20.0% vs. 5.2%, p < 0.001) was observed more frequently in male patients than in female patients.19
Defecation syncope is defined as syncope occurring or immediately after defecation. Pathy20 first reported defecation syncope, and found that 7 of 9 patients were female. Our study also showed gender difference of defecation syncope. Defecation syncope (16.3% vs. 9.3%, p < 0.001) was observed more frequently in female patients than in male patients. Kapoor, et al.21 stated that defecation syncope is not a single distinct clinical entity, because patients experienced the majority of recurrence which was not associated with defecation. However, our study showed that recurrent episodes in patients with defecation syncope were mostly associated with preceding abdominal pain and defecation sensation.
This study was retrospective in design and performed at a single tertiary referral center. Referral bias might affect the results of our study. Surveys of young adult with syncope showed that about 50% of patients did not consult a physicians.11 Therefore, this may compromise the accuracy of the data presented in Fig. 2. We evaluated only the natural history and clinical characteristics of patients with recurrent neurally mediated syncope at the time of HUT. Therefore, we did not address the pattern of the episodes with regard to symptom-free intervals in patients with recurrent neurally mediated syncope in general population.
Figures and Tables
Fig. 1
Distribution of age of first syncopal episode in 1,051 patients with recurrent neurally mediated syncope, expressed as proportions per 5-year bin of the total.
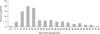
Fig. 2
Distribution of time intervals between first and last syncopal episodes in 1,051 patients with recurrent neurally mediated syncope.
References
1. Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, et al. Incidence and prognosis of syncope. N Engl J Med. 2002. 347:878–885.

2. Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003. 91:1006–1008.

3. Lipsitz LA, Wei JY, Rowe JW. Syncope in an elderly, institutionalised population: prevalence, incidence, and associated risk. Q J Med. 1985. 55:45–54.
4. Savage DD, Corwin L, McGee DL, Kannel WB, Wolf PA. Epidemiologic features of isolated syncope: the Framingham Study. Stroke. 1985. 16:626–629.

5. Grubb BP. Pathophysiology and differential diagnosis of neurocardio-genic syncope. Am J Cardiol. 1999. 84:3Q–9Q.
6. Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet. 1986. 1:1352–1355.
7. Colman N, Nahm K, van Dijk JG, Reitsma JB, Wieling W, Kaufmann H. Diagnostic value of history taking in reflex syncope. Clin Auton Res. 2004. 14:Suppl 1. 37–44.

8. Sheldon RS, Sheldon AG, Connolly SJ, Morillo CA, Klingenheben T, Krahn AD, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006. 17:49–54.
9. Natale A, Geiger MJ, Maglio C, Newby KH, Dhala A, Akhtar M, et al. Recurrence of neurocardiogenic syncope without pharmacologic interventions. Am J Cardiol. 1996. 77:1001–1003.

10. Barón-Esquivias G, Errázquin F, Pedrote A, Cayuela A, Gómez S, Aguilera A, et al. Long-term outcome of patients with vasovagal syncope. Am Heart J. 2004. 147:883–889.

11. Calkins H, Zipes DP. Libby P, Bonow RO, Mann DL, Zipes DP, Braunwald E, editors. Hypotesion and syncope. Braunwald's heart disease: a text book of cardiovascular medicine. 2007. 8th ed. Philadelphia (PA): W.B. Saunders;975–984.
12. Kim PH, Ahn SJ, Kim JS. Frequency of arrhythmic events during head-up tilt testing in patients with suspected neurocardiogenic syncope or presyncope. Am J Cardiol. 2004. 94:1491–1495.

13. Brignole M, Menozzi C, Gianfranchi L, Oddone D, Lolli G, Bertulla A. Carotid sinus massage, eyeball compression, and head-up tilt test in patients with syncope of uncertain origin and in healthy control subjects. Am Heart J. 1991. 122:1644–1651.

14. Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. 1998. 275:R1909–R1920.
15. Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004. 286:H449–H457.

16. Frey MA, Hoffler GW. Association of sex and age with responses to lower-body negative pressure. J Appl Physiol. 1988. 65:1752–1756.

17. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000. 101:862–868.
18. Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol. 2005. 289:H631–H642.

20. Pathy MS. Defeacation syncope. Age Ageing. 1978. 7:233–236.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download