Abstract
The third lineage of T helper subsets, Th17, has recently been identified as an IL-17-producing CD4+ Th cell, and its functions and regulatory mechanisms have been extensively characterized in immune responses. Functional studies have provided evidence that Th17 cells are important for the modulation of autoimmune responses, such as chronic asthma, rheumatoid arthritis, inflammatory bowel diseases, and multiple sclerosis. Murine Th17 cell differentiation is enhanced by the coordinated functions of distinct cytokines including TGFβ, IL-6, IL-21, and IL-23, whereas IL-2, IL-4, IFNγ, and IL-27 inhibit its differentiation. In addition, Th17 cells are controlled by several transcription factors such as RORγ t, IRF4, BATF, FoxP3, T-bet, PPARγ, E-FABP, and SOCSs. This review focuses on the functions and regulatory mechanisms of several transcription factors in the control of Th17 cell differentiation.
CD4+ T helper (Th) precursor cells are activated by the antigenic stimulation of T cell receptor (TCR) and are subsequently differentiated into different subsets of effector Th cells in order to boost the immune responses.1 Th1 and Th2 cells are traditionally thought to be the major subsets generated upon antigenic stimulation and produce distinct cytokine interferon γ(IFNγ) and interleukin (IL)-4, which are then involved in the elimination of intracellular and extracellular pathogens, respectively.2 Coordinated cytokine signaling induces the activation of specific transcription factors to promote lineage-specific cytokine production. While T-box-containing protein expressed in T cells (T-bet) is activated by IL-12 and IFNγ and is exclusively expressed in Th1 cell differentiation,3 GATA-binding protein 3 (GATA-3) and c-Maf are required for the chromatin remodeling and direct activation of Th2 cytokines IL-4, IL-5, and IL-13 for Th2 cell development.4,5 The balance of Th1/Th2 cells is thought to be determined by the expression ratio of T-bet/GATA-3 and is important for inducing autoimmune and allergic immune responses.6 However, the Th1/Th2 paradigm was recently shifted to the Th1/Th2/Th17/regulatory T (T-reg) hypothesis, a multi-lineage commitment from the same Th precursor cells.7,8 regulatory T cells, referred to as regulatory T cells, express forkhead box P3 (FoxP3) and suppress activated immune responses by producing transforming growth factor β(TGFβ)9,10 whereas Th17 cells induce retinoic acid-related orphan receptor γt (RORγt)-mediated IL-17 production and control the inflammatory autoimmune response.11,12 The differentiation of Th17 and T-reg cells requires the activation of TGFβ-mediated signaling, and IL-6 selectively drives Th17 cell differentiation from TGFβ-stimulated Th cells by promoting sequential activation of IL-21 and IL-23 signaling.10,13
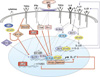
Here, we review the current understanding of the transcription factors involved in the regulation of Th17 cell differentiation, including updates of RORγt, FoxP3, and other Th17-specific transcription factors such as interferon regulatory factor 4 (IRF4), B-cell activating transcription factor (BATF), peroxisome proliferator activated receptor (PPARγ), T-bet, and suppressors of cytokine signaling (SOCS) 3 (Fig. 1).
Many scientists have reported that TGFβ and IL-6 are essential for Th17 cell differentiation.12,14,15 TGFβ, which is produced by innate immune cells, binds to its specific receptor and induces engagement of TGFβ receptor I and II with subsequent activation of receptor-associated SMADs. Activated SMADs interact with a variety of transcription factors, resulting in chromatin remodeling and modulation of gene transcription of TGFβ target genes.16 TGFβ inhibits signal transducer and activator of transcription (STAT5)-mediated IL-2 production in TCR-stimulated T cells17 and also interferes with Th1 and Th2 cell differentiation by inhibiting expression of master transcription factor T-bet and GATA-3.18,19 In addition, TGFβ increases FoxP3 expression and induces generation of T-reg cells.10 FoxP3-positive T-reg cells potently suppress cell proliferation and differentiation of Th cells by boosting TGFβ production.10 Overexpression of TGFβ in a T cell-specific manner in mice leads to the generation of T cells with regulatory functions and protects IL-2-deficient mice from developing severe systemic inflammation with autoimmunity.20,21 However, additional production of the pro-inflammatory cytokine IL-6 along with TGFβ suppresses FoxP3 expression and T-reg cell generation and simultaneously induces IL-17 production, resulting in Th17 cell differentiation.12 Consistent with this, TGFβ transgenic mice treated with myelin oligodendrocyte glycoprotein (MOG) in complete Freund's adjuvant (CFA) exhibit substantially increased Th17-mediated immune responses and aggravated experimental autoimmune encephalomyelitis (EAE),12 indicating that TGFβ plus IL-6 induces the generation of Th17 cells. Despite the importance of TGFβ function in murine Th17 cells, TGFβ is dispensable for human Th17 cell differentiation. Instead, human Th17 cells are induced by stimulation with IL-6, together with another cytokine such as IL-1, IL-21, or IL-23.15,22,23
IL-6 is a key factor for inducing human and murine Th17 cell differentiation by activating STAT3 and RORγt expression. STAT3 activation is induced not only by IL-6 but also by IL-21 and IL-23.13,24,25 While STAT3-null cells fail to express RORγt and produce a diminished level of IL-17, retroviral restoration of STAT3 rescues the IL-17 defect.26 Although it is still unclear whether STAT3 modulates RORγt gene transcription, activated STAT3 directly binds to the STAT-binding sites in the IL-17 gene promoter and increases IL-17 gene transcription.27 In addition to the functional importance of STAT3 activation in Th17 cell differentiation, RORγt has been identified as a master regulator of Th17 cell differentiation. Analogous to STAT4-mediated T-bet in Th1 cells and STAT6-dependent GATA-3 in Th2 cells, Th17 cells require activation of STAT3 and subsequent RORγt induction.11 RORγt, a spliced isoform of RORγ, is a member of the nuclear receptor superfamily, and is closely related to the retinoic acid receptor (RAR) subfamily,28 and is required for thymocyte survival and lymphoid organogenesis.29 Deficiency of RORγt results in profound Th17 deficiency and protects mice from EAE development.11 Ectopic overexpression of STAT3 in RORγt-deficient cells, and vice versa, fails to restore IL-17 production.13 This suggests that STAT3 and RORγt may each regulate the other's gene transcription and induce IL-17 expression parallel to some extent. More recently, RORα was reported as a novel Th17-specific transcription factor. Like RORγt, RORα is induced by TGFβ plus IL-6 in a STAT3-dependent manner, and promotes Th17 cell differentiation through direct activation of IL-17 gene transcription.30 Double deficiency of RORγt and RORα results in complete blockade of IL-17 production and EAE development.30
IRF4 was originally identified as a GATA-3 inducer in Th2 cell differentiation.31,32 However, IRF4-null mice exhibit impaired generation of Th17 cells in response to TGFβ and IL-6 and increased resistance to EAE.33 In addition, IRF4-deficient Th cells exhibit an intrinsic defect in the autocrine IL-21 loop and an increased population of FoxP3-mediated T-reg cells with no effect on STAT3 activation and SOCS3 expression.34 A more recent report implies that IRF4 is activated upon IL-1 signaling and is critical for early Th17 cell differentiation.35 Despite the importance of IRF4 in Th17 cell differentiation, the molecular mechanisms are unclear. The fact that IRF4 interacts with NFATp to induce IL-4 expression may suggest that IRF4 modulates NFATp-dependent IL-2 expression, which is associated with IL-17 production.36,37
The BATF is a basic leucine zipper (bZIP) transcription factor and dimerizes with Jun class factors of the activator protein-1 (AP-1) family.38-40 BATF is known to function as a potent inhibitor of AP-1-mediated gene expression via the phosphorylation of BATF.40-42 In addition, expression analysis reveals that BATF is highly expressed in hematopoietic cells and is increased in B and T cells by the activation of NF-κB in response to viral infection or IL-6-stimulation.43-47 BATF gene transcription is substantially increased in activated Th cells subsets including Th1, Th2, and Th17 cells.48 Despite its wide expression in all Th1, Th2, and Th17 cells, BATF-deficiency fails to generate IL-17 in CD4+ and CD8+ T cells in vitro and in vivo, but rather increases T-reg cell generation, thus protecting mice from EAE development.48 While the levels of RORα and RORγt expression are not sustained in BATF-deficient Th17 cells compared with those in wild type (WT) cells, enforced RORγt expression is not able to restore IL-17 production in BATF-deficient Th cells. Nevertheless, BATF synergizes with RORγt to induce IL-17 expression through direct interaction with the IL-17 gene promoter.48 Many questions regarding BATF, such as whether IL-6-induced STAT3 activation is affected by BATF deficiency and whether BATF is required for DNA binding of RORγt or IRF4, remain to be addressed in the future.49
Differentiation of FoxP3-directed T-reg cells and RORγt driven Th17 cells has been shown to be triggered by TGFβ signaling, but the Th17 differentiation program requires additional IL-6 or IL-21 cytokine signaling either to switch off FoxP3 or to switch on RORγt,10,11,15 suggesting reciprocal regulation of T-reg and Th17 cells during Th cell differentiation. It has been asked whether T-reg can be converted to Th17 in response to IL-6 and how FoxP3 and RORγt modulate each other's expression or activity.30,50,51 Interestingly, FoxP3 and IL-17 are both induced upon TGFβ stimulation.13,52 In addition, FoxP3-positive Th cells produce IL-17 in the presence of IL-6 through the activation of RORγt, whereas FoxP3 antagonizes RORγt activity in a manner dependent on SMAD4, suggesting the plasticity of T-reg cells.30 Others also report that FoxP3 inhibits IL-17 expression by antagonizing RORγt function in a TGFβ concentration-dependent manner53 or through direct interaction with RORγt.54 Like the suppressive function of FoxP3 on IL-17 expression, the Th1-specific transcription factor T-bet suppresses RORγt-mediated Th17 cell differentiation.55-57 Several functional studies indicate that T-bet suppresses RORγt expression and Th17 cell differentiation and further attenuates autoimmune responses.56,58-62 Nonetheless, the mechanism by which T-bet directly or indirectly inhibits IL-17 expression and whether T-bet antagonizes RORγt activity remain to be characterized. In addition, a T-bet-interacting transcription factor, Ets-1 positively modulates Th1 cell differentiation but inhibits Th17 cell generation.63,64 Ets-1-deficient Th cells exhibit preferential differentiation into Th17 cells and increased IL-22 and IL-23 receptor expression.64 Moreover, targeting of Ets-1 by microRNA miR-326 promotes Th17 differentiation.65 Since there is no apparent interaction between Ets-1 and IL-17 gene promoter, how Ets-1 modulates IL-17 expression must be defined in the future.
Peroxisome proliferator-activated receptorγ (PPARγ) is a nuclear receptor like RORγt and RORα and forms heterodimers with retinoid X receptors (RXRs) to bind to the gene promoter.66,67 PPARγ activation upon ligand binding is critical for the expression of genes such as adiponectin and fatty acid-binding protein (FABP) (also referred as aP2) involved in adipocyte differentiation and lipid metabolism68,69 While enforced PPARγ expression induces adipocyte differentiation from fibroblasts, PPARγ-deficiency attenuates white adipose tissue development.70 Although PPARγ functions as a master transcriptional regulator for adipocyte differentiation, the anti-inflammatory activity of PPARγ is also well-characterized.71-73 The anti-inflammatory function of PPARγ is mediated through the inhibition of both maturation and function of dendritic cells and macrophages.74,75 More precisely, the ligand-binding domain of PPARγ is sumoylated upon ligand activation and prevents the removal of repressor complexes composed of nuclear receptor corepressor and histone deacetylase-3, thus resulting in sustained repressor complex-induced silencing of pro-inflammatory cytokine genes.76,77 In addition to the modulation of macrophage function, PPARγ modulates T cell activity by inhibiting IL-2 production in T cell receptor-stimulated Th cells78 and by suppressing Th2 cell differentiation.79 Therefore, PPARγ ligands including endogenous and synthetic agonists such as linoleic acid, prostaglandin J2, and thiazolidinediones have been extensively studied due to the interest in treating inflammatory diseases.71,80,81 A recent study demonstrates that PPARγ is an intrinsic suppressor for Th17 cell generation.82 PPARγ activation is thought to prevents removal of repressor complexes from RORγt gene promoter, thus suppressing RORγt expression and RORγt-induced Th17 cell differentiation in an intrinsic manner. Moreover, human multiple sclerosis patients are impressively susceptible to PPARγ-mediated suppression of Th17 cell development, strongly asserting PPARγ as a promising target for specific immunointervention in autoimmune disorders.82
In contrast to the suppressive function of adipogenic PPARγ, epidermal FABP (E-FABP) is characterized as a positive modulator of IL-17 production in Th cells.83 FABP-deficiency contributes to the protection from EAE development,84 which has been explained by the reduced level of pro-inflammatory cytokines in macrophages and dendritic cells.85 Moreover, FABP-deficient Th cells express increased amounts of PPARγ and subsequently suppress IL-17 production; however, this can be reversed by treatment with the PPARγ antagonist, GW9662.83 More detailed molecular mechanisms of E-FABP have yet to be characterized.
The SOCS inhibit STAT-mediated cytokine signaling.86,87 Since SOCS1 suppresses both IFNγ- and IL-4-mediated Th1 and Th2 cell differentiation, genetic ablation of SOCS1 results in unconditional hyperactivation of T cells.88 In addition, T cell-specific SOCS1-conditional knockout mice exhibit attenuated Th17 cell generation and induced hyperactivation of SOCS3 in Th cells,89 suggesting that SOCS1 as a transcriptional activator for Th17 cell development. Activated SOCS3 is known to selectively suppress STAT-3 activation induced by IL-6, granulocyte-colony stimulating factor (GCSF), and leptin,90,91 whereas deficiency of SOCS3 increases TGFβ production and simultaneously enhances Th17 cell development.27,92 It is also reported that TGFβ inhibits SOCS3 gene transcription and prolongs STAT3 activation during Th17 cell differentiation.93
With the recent reports of the functions of IL-17-producing Th17 cells in autoimmune responses and the regulatory mechanisms for Th17 cell differentiation, a new paradigm of Th cell differentiation has been established. The Th1/Th2 paradigm is mainly shifted to a Th1/Th2/ Th17/T-reg program. This review describes the function and potential mechanisms of transcription factors critical for the regulation of Th17 cell differentiation and also includes interconnection among transcription factors such as Th1-specific T-bet, T-reg-limited FoxP3, and Th17-specific RORγt . These transcription factors have prevalent roles in determining lineage commitment through interaction with cytokine gene promoters and/or other lineage-specific transcription factors. Targeting these transcription factors, as well as signature cytokines, may be beneficial for controlling several autoimmune diseases, although research is currently underway to identify the detailed mechanisms.
Figures and Tables
Fig. 1
Cytokine signaling and transcription factors in the regulation of Th17 cell differentiation. TCR stimulation activates gene expression of general transcription factors such as NFAT, AP-1, and NF-κB, and induces Th cell activation and proliferation. BATF is activated upon TCR stimulation and stimulates IL-17 gene transcription. TGFβ stimulation induces both FoxP3 and RORγt (also RORa) activation. High concentrations of TGFb increase FoxP3 through the activation of SMAD4 and subsequently induce TGFb production and simultaneously suppress RORγt activity and Th17 cell differentiation. However, the presence of cytokine IL-6 or IL-21 activates STAT3 and induces gene expression of the IL-21 and IL-23 receptor, activating positive IL-21 autocrine regulation for Th17 cell differentiation. In addition, IL-1 induces IRF4 or epidermal FABP4, which in turn induces IL-17 gene transcription. While T-bet and Ets-1 antagonize RORγt activity and thus function as suppressors of Th17 cell development, PPARγ intrinsically suppresses IL-17 gene transcription by blocking the activation-induced removal of repressor complexes from the IL-17 gene promoter. SOCS1 and SOCS reciprocally modulate Th17 cell differentiation. TCR, T cell receptor; NFAT, nuclear factor of activated T cells; AP, activator protein; BATF, B cell-activating transcription factor; IL-17, interleukin-17; TGFβ, transforming growth factor β; RORγt, retinoic acid-related orphan receptor γt; STAT, signal transducer and activator of transcription; IRF-4, interferon-inducible factor-4; E-FABP, epidermal-fatty acid-binding protein; PPARγ, peroxisome proliferator activated receptor γ; SOCS, suppressors of cytokine signaling.
ACKNOWLEDGEMENTS
This work was supported by the R&D program (A080908) of KHIDI and partly by the NCRC program (R15-2006-020) funded by MEST.
References
1. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.
3. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000. 100:655–669.

4. Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998. 9:745–755.

5. Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998. 188:1859–1866.

6. Kiwamoto T, Ishii Y, Morishima Y, Yoh K, Maeda A, Ishizaki K, et al. Transcription factors T-bet and GATA-3 regulate development of airway remodeling. Am J Respir Crit Care Med. 2006. 174:142–151.

7. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007. 25:821–852.
8. Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007. 19:652–657.

9. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.
10. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. 198:1875–1886.

11. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006. 126:1121–1133.

12. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006. 441:235–238.

13. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007. 8:967–974.

14. Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006. 441:231–234.

15. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006. 24:179–189.

16. Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999. 18:1280–1291.

17. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007. 26:371–381.

18. Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002. 195:1499–1505.

19. Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol. 2000. 165:4773–4777.

20. Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007. 178:179–185.

21. Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J Immunol. 2007. 178:172–178.

22. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007. 8:950–957.

23. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007. 8:942–949.

24. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007. 448:480–483.

25. Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007. 282:9358–9363.
26. Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007. 178:4901–4907.

27. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006. 103:8137–8142.
28. Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003. 195:81–90.
29. Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000. 288:2369–2373.

30. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008. 28:29–39.

31. Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002. 195:1003–1012.
32. Lohoff M, Mittrücker HW, Prechtl S, Bischof S, Sommer F, Kock S, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci U S A. 2002. 99:11808–11812.

33. Brüstle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007. 8:958–966.

34. Huber M, Brüstle A, Reinhard K, Guralnik A, Walter G, Mahiny A, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008. 105:20846–20851.

35. Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009. 30:576–587.

36. Littman DR, Sun Z, Unutmaz D, Sunshine MJ, Petrie HT, Zou YR. Role of the nuclear hormone receptor ROR gamma in transcriptional regulation, thymocyte survival, and lymphoid organogenesis. Cold Spring Harb Symp Quant Biol. 1999. 64:373–381.
37. Chen Z, Laurence A, O'Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007. 19:400–408.

38. Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001. 20:2438–2452.

39. Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene. 1995. 11:2255–2265.
40. Echlin DR, Tae HJ, Mitin N, Taparowsky EJ. B-ATF functions as a negative regulator of AP-1 mediated transcription and blocks cellular transformation by Ras and Fos. Oncogene. 2000. 19:1752–1763.

41. Williams KL, Nanda I, Lyons GE, Kuo CT, Schmid M, Leiden JM, et al. Characterization of murine BATF: a negative regulator of activator protein-1 activity in the thymus. Eur J Immunol. 2001. 31:1620–1627.

42. Deppmann CD, Thornton TM, Utama FE, Taparowsky EJ. Phosphorylation of BATF regulates DNA binding: a novel mechanism for AP-1 (activator protein-1) regulation. Biochem J. 2003. 374:423–431.

43. Li J, Peet GW, Balzarano D, Li X, Massa P, Barton RW, et al. Novel NEMO/IkappaB kinase and NF-kappa B target genes at the pre-B to immature B cell transition. J Biol Chem. 2001. 276:18579–18590.

44. Hasegawa T, Zhou X, Garrett LA, Ruteshouser EC, Maity SN, de Crombrugghe B. Evidence for three major transcription activation elements in the proximal mouse proalpha2(I) collagen promoter. Nucleic Acids Res. 1996. 24:3253–3260.

45. Meyer NP, Johansen LM, Tae HJ, Budde PP, Williams KL, Taparowsky EJ. Genomic organization of human B-ATF, a target for regulation by EBV and HTLV-1. Mamm Genome. 1998. 9:849–852.

46. Johansen LM, Deppmann CD, Erickson KD, Coffin WF 3rd, Thornton TM, Humphrey SE, et al. EBNA2 and activated Notch induce expression of BATF. J Virol. 2003. 77:6029–6040.

47. Senga T, Iwamoto T, Humphrey SE, Yokota T, Taparowsky EJ, Hamaguchi M. Stat3-dependent induction of BATF in M1 mouse myeloid leukemia cells. Oncogene. 2002. 21:8186–8191.

48. Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009. 460:405–409.

49. Martinez GJ, Dong C. BATF: bringing (in) another Th17-regulating factor. J Mol Cell Biol. 2009. 1:66–68.

50. Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008. 38:3274–3281.
51. Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008. 20:1361–1368.
52. Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008. 29:44–56.
53. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008. 453:236–240.
54. Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, et al. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008. 283:17003–17008.
55. Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, et al. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006. 108:1595–1601.
56. Fujiwara M, Hirose K, Kagami S, Takatori H, Wakashin H, Tamachi T, et al. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J Allergy Clin Immunol. 2007. 119:662–670.
57. Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007. 178:1341–1348.
58. Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG, Metzger DW. Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J Immunol. 2009. 183:5293–5300.
59. Rutitzky LI, Smith PM, Stadecker MJ. T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur J Immunol. 2009. 39:2470–2481.
60. Abromson-Leeman S, Bronson RT, Dorf ME. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J Neuroimmunol. 2009. 215:10–24.
61. Guo S, Cobb D, Smeltz RB. T-bet inhibits the in vivo differentiation of parasite-specific CD4+ Th17 cells in a T cell-intrinsic manner. J Immunol. 2009. 182:6179–6186.
62. Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006. 203:2009–2019.
63. Grenningloh R, Kang BY, Ho IC. Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J Exp Med. 2005. 201:615–626.
64. Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007. 204:2825–2835.
65. Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009. 10:1252–1259.

66. Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000. 5:545–555.

67. Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, et al. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994. 22:5628–5634.

68. Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005. 102:6207–6212.
69. Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005. 115:2244–2256.

70. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999. 4:611–617.
71. Na HK, Surh YJ. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands as bifunctional regulators of cell proliferation. Biochem Pharmacol. 2003. 66:1381–1391.

72. Chen CW, Chang YH, Tsi CJ, Lin WW. Inhibition of IFN-gamma-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor gamma agonist, 15-deoxydelta 12,14-prostaglandin J2, involves inhibition of the upstream Janus kinase/STAT1 signaling pathway. J Immunol. 2003. 171:979–988.

73. Schmidt S, Moric E, Schmidt M, Sastre M, Feinstein DL, Heneka MT. Anti-inflammatory and antiproliferative actions of PPAR-gamma agonists on T lymphocytes derived from MS patients. J Leukoc Biol. 2004. 75:478–485.

74. Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, et al. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol. 2007. 178:2122–2131.
75. Szatmari I, Töröcsik D, Agostini M, Nagy T, Gurnell M, Barta E, et al. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007. 110:3271–3280.

76. Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005. 437:759–763.

77. Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007. 28:551–558.
78. Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000. 164:1364–1371.

79. Won HY, Min HJ, Ahn JH, Yoo SE, Bae MA, Hong JH, et al. Anti-allergic function and regulatory mechanisms of KR62980 in allergen-induced airway inflammation. Biochem Pharmacol. 2010. 79:888–896.

80. Meyers CD, Kashyap ML. Pharmacologic elevation of high-density lipoproteins: recent insights on mechanism of action and atherosclerosis protection. Curr Opin Cardiol. 2004. 19:366–373.

81. Xu J, Barger SW, Drew PD. The PPAR-gamma Agonist 15-Deoxy-Delta-Prostaglandin J(2) Attenuates Microglial Production of IL-12 Family Cytokines: Potential Relevance to Alzheimer's Disease. PPAR Res. 2008. 2008:349185.
82. Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009. 206:2079–2089.
83. Li B, Reynolds JM, Stout RD, Bernlohr DA, Suttles J. Regulation of Th17 differentiation by epidermal fatty acid-binding protein. J Immunol. 2009. 182:7625–7633.
84. Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, et al. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol. 2007. 179:313–321.
85. Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw IA, et al. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J Immunol. 2006. 177:7794–7801.
86. Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003. 4:1169–1176.

87. Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007. 7:454–465.
88. Chinen T, Kobayashi T, Ogata H, Takaesu G, Takaki H, Hashimoto M, et al. Suppressor of cytokine signaling-1 regulates inflammatory bowel disease in which both IFNgamma and IL-4 are involved. Gastroenterology. 2006. 130:373–388.

89. Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008. 180:3746–3756.

90. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004. 10:739–743.

91. Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003. 4:551–556.
92. Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006. 203:1021–1031.

93. Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009. 183:97–105.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download