Abstract
The clinical spectrum of infections caused by non-typhoid Salmonella spp. includes gastroenteritis, enteric fever, bacteremia, and extraintestinal localized complications, especially in immunocompromised hosts. Here we report a patient with severe aplastic anemia developing left iliopsoas abscess caused by non-typhoid Salmonella (NTS), which was successfully treated by prolonged antibiotic treatment and repeated debridement. Our data indicate that aplastic anemia is a risk factor for infection caused by NTS.
Infections caused by Salmonella spp. are common and the incidence is increasing in many countries.1 Not uncommonly, non-typhoid Salmonella (NTS) causes infections in immunocompromised hosts, such as patients with malignancies, acquired immunodeficiency syndrome (AIDS), diabetes mellitus, and those receiving corticosteroid therapy.2 The clinical spectrum of NTS infections ranges from asymptomatic chronic carrier state, gastroenteritis, enteric fever, bacteremia to extraintestinal localized complications such as fatal endovascular infection.3 However, psoas abscess due to NTS has rarely been reported.4,5 We here describe a patient with severe aplastic anemia (SAA) who developed psoas abscess caused by NTS. To the best of our knowledge, this is the first report of psoas abscess caused by NTS in such hosts.
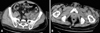
In early November of 2005, a 52-year-old man with a three-month history of severe aplastic anemia (SAA) developed left hip pain that was initially treated as muscle strain. His baseline hemogram remained low (white blood cell, 2.23×103/µL; hemoglobin, 6.8 gm/dL; platelet, 14×103/µL) even after therapy with antithymocyte globulin (ATG), cyclosporine, and corticosteroid for his SAA. Fever with progressive dyspnea developed in the following three weeks with septic shock and respiratory failure (PaO2 = 56.4 mmHg on 60% supplemental oxygen) then ensued. The patient was put on mechanical ventilation assistance. At that time, the hemogram showed anemia (hemoglobin 6.7 gm/dL), leucopenia (total leukocyte count, 0.74×103/µL, with 78% of neutrophils and 16% of lymphocytes), and severe thrombocytopenia (platelet 7×103/µL). Elevated C-reactive protein (CRP, 26.76 mg/dL) and acute renal failure (creatinine 4.0 mg/dL) were also noted. The chest radiograph was unremarkable. Cefepime (2 gm, every 12 hours, intravenously) and metronidazole (250 mg, every 6 hours, intravenously) were administrated empirically along with granulocyte-colony stimulating factor (G-CSF) (300 µg, every day). The computed tomography (CT) of the abdomen and pelvis disclosed a hypodense lesion, measuring 2.5×2 cm in size, in the left psoas-iliacus muscle (Fig. 1). Both of the first two sets of blood culture yielded Salmonella O9 (Group D). The bacterial culture of the pus-like aspirate via CT guided aspiration of suspected psoas abscess also yielded Salmonella O9 (Group D). With a two-week course of G-CSF and antibiotics, hypoxemia and inflammatory surrogates improved (CRP, 3.65 mg/dL), and the blood culture became sterile. However, newly-onset left calf pain with redness and swelling developed on hospital day 14. A CT scan of the lower limbs revealed an abscess and deep fascia thickening at the left calf muscle area. Open drainage of the left calf abscess and repeated debridement for the left psoas-iliacus abscess were performed. Osteomyelitis was noted during the operation. Ceftriaxone (2 gm every 12 hours, intravenously) and metronidazole (250 mg every 6 hours, intravenously) were maintained thereafter. The patient then became afebrile and was discharged with ciprofloxacin (750 mg, every 12 hours, orally) after a three-month hospitalization. The patient had three more admissions later for wound debridement and parenteral antibiotic therapy after the first discharge due to recurrent fever and intolerable hip pain in the subsequent nine months. Ciprofloxacin was discontinued after 10 months of use. To date, the patient remains in good condition. This case demonstrates a special condition that required long-term antibiotic use after focal NTS infection. The entire therapeutic course is summarized in Fig. 2.
Among the five clinical manifestations of salmonellosis, acute gastroenteritis is the most common presentation (up to 75%). Asymptomatic chronic carrier state, enteric fever, bacteremia, and extraintestinal localized complications of the meninges, joints, wounds, or gall bladder account for the remaining cases.6 Generally, pyogenic iliopsoas abscess can be divided into two categories. First, the abscess is secondary to the hematogenous spread of Staphylococcus aureus (SA).7 The second category consists of abscess formation subsequent to infections of the neighboring organs and/or tissues. Mixed enteric bacteria predominate in the latter group.8 However, psoas abscess caused by NTS is extremely rare. Patients with underlying diseases such as diabetes, cancer, human immunodeficiency virus (HIV) infection, reticuloendothelial blockade (e.g., malaria, sickle cell disease, or bartonellosis), and suppressed immunity of all kinds are at increased risk for NTS infections.2 Other pathogens causing psoas muscle abscess - including Mycobacterium avium intracellulare, tuberculosis, and disseminated nocardiosis9,10 - have also emerged, especially among patients with HIV infection.11
A previous report also pointed out that debridement was indicated for more effective control of psoas abscess.8 The diagnosis of iliopsoas abscess is often delayed due to vague initial presentations, low index of suspicion among physicians, and a lack of specific physical signs in this anatomic area as demonstrated in the present case. It is not uncommon for patients to experience symptoms for weeks to months before a proper diagnosis is ultimately made.12 A CT scan is probably the most sensitive diagnostic method and CT-guided pecutaneous catheter drainage could further help confirm the diagnosis and obtain appropriate therapeutic interventions.13,14
Our patient was the first case of NTS psoas muscle abscess with an underlying disease of SAA. The combination of ATG and cyclosporine as immunosuppressive reagents is the current standard therapy in SAA.15 Immunosuppressive therapy (IST) makes the patients with aplastic anemia even more susceptible to life-threatening infections. In addition to immunocompromized status, bleeding tendency would complicate any surgical intervention and further obscure optimal surgical timing in this group of patients. Besides, local hematoma related to bleeding tendency would make infection control even more difficult. Therefore, maintenance of adequate platelet count by component therapy before surgical interventions to prevent formation of local hematoma is paramount in such patients.
In summary, we demonstrated the first case of psoas abscess caused by NTS in a patient with SAA. Psoas abscess caused by NTS should be suspected when an immunocompromised patient presents as hip or back pain in the presence of severe infection. Early surgical intervention is critical to control the infection despite severe thrombocytopenia in this group of patients.
Figures and Tables
References
1. Miller S, Pegues D. Mandell GL, Bennett JE, Dolin R, editors. Salmonella species, including Salmonella typhi. Mandell, Douglas, and Bennet's principles and practice of infectious diseases. 2000. 5th ed. Philadelphia: Churchill Livingstone;2344–2363.
2. Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001. 32:263–269.
3. Wang JH, Liu YC, Yen MY, Wang JH, Chen YS, Wann SR, et al. Mycotic aneurysm due to non-typhi salmonella: report of 16 cases. Clin Infect Dis. 1996. 23:743–747.
4. Laguna P, Moya M. [Abscess of the psoas muscle: analysis of 11 cases and review of the literature.]. Enferm Infecc Microbiol Clin. 1998. 16:19–24.
5. Liao YS, Shih HN, Hsu RW. Salmonella psoas abscess--a case report. Changgeng Yi Xue Za Zhi. 1995. 18:170–175.
6. Heyd J, Meallem R, Schlesinger Y, Rudensky B, Hadas-Halpern I, Yinnon AM, et al. Clinical characteristics of patients with psoas abscess due to non-typhi Salmonella. Eur J Clin Microbiol Infect Dis. 2003. 22:770–773.
8. Santaella RO, Fishman EK, Lipsett PA. Primary vs secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch Surg. 1995. 130:1309–1313.
9. Corti M, Solari R, De Carolis L, Cangelos D, Bianchi M, Negroni R. Disseminated nocardiosis with psoas abscess in a patient with AIDS: first reported case. Rev Inst Med Trop Sao Paulo. 2008. 50:131–133.

11. Navarro López V, López García F, González Escoda E, Gregori Colomé J, Muñoz Pérez A. Psoas abscess in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 2004. 23:661–663.

12. Hamano S, Kiyoshima K, Nakatsu H, Murakami S, Igarashi T, Ito H. Pyogenic psoas abscess: difficulty in early diagnosis. Urol int. 2003. 71:178–183.

13. Cantasdemir M, Kara B, Cebi D, Selcuk ND, Numan F. Computed tomography-guided percutaneous catheter drainage of primary and secondary iliopsoas abscesses. Clin Radiol. 2003. 58:811–815.

14. Muttarak M, Peh WC. CT of unusual iliopsoas compartment lesions. Radiographics. 2000. 20(Spec No):S53–S66.
15. Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008. 15:162–168.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download