Abstract
Purpose
Preoperative identification of the sentinel lymph node (SLN) in gastric cancer (GC) patients may have great advantages for the minimally invasive treatment. This study was performed to evaluate the possibility of preoperative SLN detection using CT lymphography.
Materials and Methods
Fourteen patients with early GC were enrolled. CT images were obtained before and at 1, 3, and 5 minutes after endoscopic submucosal peritumoral injection of 2 mL iopamidol. For patients with clearly identified SLNs, to make comparisons with the CT lymphography results, intraoperative SLN detection was performed using subserosally injected Indocyanine green (ICG) lymphography and ex vivo ICG and iopamidol lymphography using mammography was also performed.
Results
CT lymphography clearly visualized draining lymphatics and SLNs in 4 (28.6%) out of 14 patients. All clearly visualized SLNs (one to three SLNs per patient) under preoperative imaging were detected in the same location by intraoperative ICG lymphography and ex vivo ICG and iopamidol lymphography using mammography. All preoperative SLN detections were observed with the primary tumors in the lower third of the stomach.
Recent trends in gastric cancer treatment involve improving survival after curative surgery by detecting early stage cancers. They also include improving the quality of life after treatment by reducing the extent of surgery.1-5 To improve quality of life, various modifications such as endoscopic submucosal dissection and partial gastrectomy with limited lymphadenectomy have been applied for gastric cancer treatments that are consistent with tumor biology.4-8 These minimally invasive treatments for gastric cancers have received considerable attention for their better post-treatment outcomes compared with conventional radical surgery such as gastrectomy with extended lymphadenectomy.9,10
The general assumption behind adopting limited lymphadenectomy for early gastric cancer is that regional or systemic lymphadenectomy is appropriate for clinically suspicious or pathologically proven metastases to regional lymph nodes.11 Thus, when there is no possibility of metastases to regional lymph nodes, surgery without lymphadenectomy or with limited lymphadenectomy should be sufficient for selected patients.12
The hypothesis of the sentinel lymph node (SLN) methodology is that a metastasis in the SLN could indicate metastases in other lymph nodes. In contrast, a negative SLN means the rest of the lymph nodes are tumor-free. In patients with negative SLNs, lymphadenectomy may be unnecessary for certain tumor types.13,14 Therefore, adopting the SLN concept is appropriate to reduce the extent of lymphadenectomy in gastric cancer, just as with solid tumors such as breast cancer and melanoma.15
However, there are still many problems with the current methods of SLN detection using vital dyes and/or radioactive tracers during surgery.15 Identifying SLNs in gastric cancer patients with a preoperative study can greatly benefit surgeons and patients. However, no reliable method for preoperatively detecting the SLN in gastric cancer has been established. Thus, the purpose of this study was to evaluate the possibility of preoperative SLN detection using CT lymphography.
Fourteen patients with early gastric cancer were endoscopically injected with radio-contrast and were examined with a CT scan for the purpose of SLN detection the day prior to surgery. Informed consent was obtained from all patients prior to examination and surgery, in accordance with the protocol approved by the ethical committee at our institution. The Institutional Review Board of our institution approved this study. The patient selection criteria for the study were as follows: 1) patients with biopsy-proven adenocarcinomas diagnosed as having early gastric cancer by upper endoscopy with endoscopic ultrasonography and 2) no history of drug allergy. Demographic and pathologic characteristics of the patients enrolled are shown in Table 1.
The day before the surgery, just before endoscopic injection of radio-contrast, precontrast CT images from the diaphragmatic dome to the level of the iliac crest were obtained while patients were in the supine position by using a multisection CT scanner (16-channel multi-detector row CT) (Sensation 16, Siemens Medical Solutions, Forchheim, Germany). Upper endoscopy was performed by an attending gastroenterologist, who has 4-years experience of performing upper endoscopy as a specialist, at the CT scanning table because CT images had to be taken within a short period of time after the contrast injection (Fig. 1A). End-viewing fiberoptic panendoscopes (GIF-Q260, GIF-H260; Olympus, Tokyo, Japan) were used after intravenously administering 5 mg of midazolam to the patient (Taro Pharmaceutical International, Yakum, Israel) using standard practices. Endoscopic injection of radio-contrast was performed using a puncture needle which was introduced endoscopically. A total of 2 mL of undiluted iopamidol (Iopamiron 370; Bayer-Schering, Berlin, Germany) was gently administered into the submucosa at four points adjacent to the tumor (with a 0.5 mL dose at each point) (Fig. 1B). CT lymphography was taken before and at 1, 3, and 5 minutes after contrast injection with the following parameters: 120 kV; 350 mA; detector collimation; 0.75 mm, matrix, 512×512; and reconstruction thickness, 1 mm (Fig. 1C). A radiologist, who has 8-years experience of specialty in hollow viscus radiology, placed regions of interest in each SLN using a CT workstation for estimating the degree of contrast enhancement. Lymph node enhancement was judged to be positive if the attenuation of a node on post-contrast images was increased by more than 30 Hounsfield units compared with the attenuation of the node on pre-contrast images.16
Intraoperative lymphatic mapping using a vital dye and SLN biopsy was performed only for those patients who showed clear visualization of SLNs in CT lymphography. Immediately after laparotomy, 2 mL of 0.63% indocyanine green solution (ICG, Diagnogreen; Dai-Ichi Pharm Co. Ltd., Tokyo, Japan) was injected into the subserosal layer at four points adjacent to the tumor. All lymph nodes that were stained green within 5 minutes after the dye injection were regarded as SLNs.
After gastrectomy with en bloc extended lymph node dissection, ex vivo detection of the SLN was performed. To match the CT lymphography results with introperative SLN detection, an X-ray mammography of the specimen was obtained after peritumoral submucosal injection of a mixture of iopamidol and ICG for ex vivo detection of the SLN at a mammography suite. All lymph nodes that were stained green were regarded as SLNs, and we examined whether they were enhanced by using the mammography.
Immediately after iopamidol lymphography using mammography, SLNs were picked up and the other regional lymph node groups were removed from the stomach. All lymph nodes were grouped according to the Japanese classification of Gastric Carcinoma.17 For all lymph nodes dissected, including SLNs, one section from each paraffin-embedded specimen was stained conventionally with hematoxylin and eosin and examined by pathologists.
CT lymphography clearly visualized draining lymphatics and SLNs within 5 minutes after contrast injection in 4 (28.6%) out of 14 patients (cases 3, 5, 7, and 9). CT lymphography failed to visualize the draining lymphatics and reliable SLNs in the ten remaining patients. The results are summarized in Table 1 and Fig. 3.
In all four patients with successful preoperative detection of the SLN, the primary tumor was located in the lower third of the stomach. The stations of SLN detected were in the perigastric location. The SLN was identified in a single lymph node station in three patients: in the infrapyloric area (station #6) for tumors located at the posterior wall of the antrum and at the greater curvature of the lower body (cases 3 and 9), and at the lesser curvature area of the lower body (station #3) for a lesion located at the lesser curvature of the lower body (case 7). For another patient with a lesion located in the lesser curvature side of the lower body (case 5), SLNs were located at lymph nodes along lesser and greater curvature regions (stations #3 and #4).
Among the four patients who underwent successful preoperative imaging of the SLN, all SLNs (one to three nodes per patient) were found intraoperatively by submucosal injection of ICG at the same location under CT lymphography guidance. And iopamidol lymphography using mammography also detected the same SLNs ex vivo. Ex vivo iopamidol lymphography with mammography clearly visualized the lymphatic flow as ICG (Fig. 2). No lymph node metastases were identified in SLNs as well as non-SLNs upon pathologic examination using hematoxylin-eosin staining in all four patients.
In the remaining ten patients, CT lymphography failed to visualize the draining lymphatics connected to the SLNs. One patient showed two metastatic lymph nodes in station #3 while the other nine patients showed no lymph node metastasis.
While tumors were located in the lower body in all four patients who underwent successful preoperative imaging of SLNs, preoperative detection of the SLN using CT lymphography was not successful in patients with a primary lesion in the mid or upper third of the stomach or with an ulcer in the primary lesion (Fig. 3) (Table 1).
In this study, we found that SLN detection using CT lymphography showed potential as a useful tool in the context of a minimally invasive treatment for early gastric cancer, although a less than 30% SLN detection rate was demonstrated.
Although there is not enough evidence of application of sentinel node concept for gastric cancer patients, recent studies have shown that sentinel lymph node navigation during gastrectomy can be an alternative for conventional extended gastrectomy for gastric cancer patients, provided that appropriate indications are employed.13-15,19 Currently, SLN detection is performed during an operation using various types of vital dye, radioisotopes, or dual methods.14,18,19 Vital dyes as tracers for SLN detection have the disadvantages of hypersensitivity, time limitations for detecting SLN, and difficulty in detecting deep-seated SLN.15 When dyes are used for SLN detection in gastric cancer, it is difficult to conduct this procedure during laparoscopy, and intraoperative endoscopy is needed for dye injection.20 Radioisotope also has problems associated with radiation hazard, the need for a gamma detector, imprecise lymphoscintigraphy information, and a shine-through effect.15 Furthermore, these intraoperative procedures for detecting SLNs make for a longer operation. Theoretically, SLN detection using CT lymphography has the advantages of avoiding radiation hazard and it allows medical personnel-including operating surgeons - to obtain accurate anatomical information, thereby eliminating additional operative procedure and reducing the operative time,21-23 although additional radiation exposure during CT lymphography does occur because CT lymphography is additionally performed after the routine preoperative CT for staging.
Although there is controversy regarding SLN detection after excisional biopsy for breast cancer and melanoma, the previous biopsy does not affect the accuracy of SLN detection. However, SLN detection after a wide excision of the primary lesion may decrease the accuracy of SLN detection because the destruction of lymphatic channels by previous surgical procedures may change the original lymphatic drainage pattern.24 The lymphatic drainage of the stomach is multidirectional.25 Usually, endoscopic submucosal dissection ESD is performed with a circumferential margin of at least 1 cm, if possible.26 Due to the possibility of different lymphatic drainage directions within different areas of the stomach, SLN navigation after ESD may not detect the SLN of the primary lesion already resected by ESD, although Abe, et al.27 have reported successful application of laparoscopic SLN navigation after ESD for five patients. Therefore, if pretreatment SLN detection using CT lymphography were to become possible, SLN navigation could be easily applied to the patients laparoscopically after endoscopic submucosal dissection. Because a CT scan is mandatory before ESD for every patient, SLN basin dissection may be possible using information obtained from CT lymphography.20
However, we identified SLNs only 30% with preoperative CT lymphography with iopamidol. The low detection rate of CT lymphography using iopamidol may be explained as follows: 1) The water soluble characteristics of iopamidol may affect rapid wash-out of interstitially injected iopamidol after diffusion into the lymphatic stream, thus causing the detection rate to depend on the timing of the CT scans in respect of vital dyes; 2) The use of non-diluted iopamidol, due to the dependence of the amount of contrast drained to the lymph node on the injected iodine dose, causes difficulty in discriminating an SLN adjacent to a primary lesion from the primary lesion itself because of beam-hardening artifacts in the context of the shine-through effect of the isotope method28; and 3) Organ-specific characteristics of the stomach in terms of SLN detection may influence the low detection rate unlike the 100% detection rate of esophageal cancer SLN detection by CT lymphography.21 In addition, the low detection rate might be affected by tumor locations. As shown in this study, detection of SLNs by CT lymphography was successful only in patients with primary lesions in the lower stomach.
The peak nodal CT attenuation after interstitial contrast injection is known to be 1 minute.21 This short optimal window for CT scanning may be related to the low detection rate of SLNs in this study, although we performed endoscopic injection of iopamidol at the CT table. Therefore, development of new radio-contrast that has the characteristics of a radioisotope and uptake by lymph nodes over a wide CT scanning time-window may be a solution to enabling a higher detection rate. Optimization of CT parameters to decrease beam hardening artifacts may be another approach to increasing the detection rate. Other technical difficulties of CT lymphography are 1) the CT room is a difficult environment for endoscopic procedure, 2) the endoscopist did not reach the learning curve at that time, and 3) technical difficulty to adjust the injection harmony with CT scanning.
Not only the technical aspects of CT lymphography, but also the tumor characteristics, such as the anatomical location and the presence of ulcers, are important factors in achieving a higher rate of SLN detection in gastric cancer. The detection rate of SLNs is decreased in tumors located in the mid or upper third,29 and SLN mapping is not useful when there is altered or destroyed lymphatic drainage.30 Our failure to detect SLNs in patients with a primary lesion in the mid or upper third of the stomach or with an ulcer in the primary lesion may be related to their tumor characteristics. Therefore, proper selection criteria based on tumor characteristics may be necessary for the application of SLN navigation in gastric cancer patients.
In this study, there was a patient with lymph node metastasis but CT lymphography failed in that patient. Because intraoperative SLN detection using ICG and ex vivo iopamidol lymphography using mammography were performed only for those patients who showed clear visualization of SLNs in CT lymphography, we cannot explain the accurate reason of non-visualization of lymph node by CT-lymphography which had been identified by histopathologic examination. However, non-visualization of the metastatic lymph node by CT lymphography in our study does not mean than CT lymphography is not a reliable method. It may come from a low detection rate of iopamidol CT lymphography.
This study examined the SLN intraoperatively only for those patients who showed clear visualization of SLNs in CT lymphography. Thus, we cannot fully evaluate the reasons of SLN detection failure in ten patients. Furthermore, all four patients with successfully detected SLNs showed no lymph node metastasis. Because of node negativity in all SLNs detected, it was impossible to evaluate positive predictive value, false negative rate, etc. Therefore, for the thorough and comprehensive evaluation of the role of CT lymphography for SLN detection in gastric cancer patients, complete SLN examinations of the patients either with or without SLNs visualized in CT lymphography and enough number of patients either with or without lymph node metastasis should be enrolled to evaluate the detection rate, accuracy, false negative, and so on. To show that nodes which are marked by CT-lymphography represent sentinel nodes, several early gastric cancers with lymph node metastasis should be included in cases with SLN detected by CT lymphography. However, as shown in Fig. 2, among the four patients who underwent successful preoperative imaging of the SLN, all SLNs were found intraoperatively by submucosal injection of ICG at the same location under CT lymphography guidance. And iopamidol lymphography using mammography also detected the same SLNs ex vivo. Ex vivo iopamidol lymphography with mammography clearly visualized the lymphatic flow as ICG (Fig. 2). Thus, we can confirm that SLNs detected by CT-lymphography is SLNs detected by dye method using ICG, although it is not proved that SLN detected CT lymphography can actually concord with metastatic lymph node.
Because we only focused on the technical possibility of CT lymphography in gastric cancer patients, we cannot validate the clinical feasibility of CT lymphography. We only found potential possibility of SLN detection by CT lymphography with a low detection rate. To show the clinical feasibility and to provide a clinical significance of SLN detection using CT lymphography, the low SLN detection rate associated with CT lymphography should be solved. If over 90% of SLN detection rate by CT lymphography is achieved by developing better protocols and contrast system, evaluation of the clinical feasibility of CT lymphography for patients with gastric cancer and the determination of the extent of lymph node detection can be reached. Although this study is a pilot study to identify the possibility of preoperative SLN detection using CT lymphography with a very small number of patients, we found potential of CT lymphography for preoperative SLN detection. And to our knowledge, this is the first study of preoperative SLN detection using CT lymphography in gastric cancer patients.
In conclusion, from the results of this study, CT lymphography with radio-contrast showed potential as a method of preoperative SLN detection for GC although the SLN detection rate was less than 30%. For better results, the development of a radio-contrast that allows a wide time-window for CT scanning and complementary methods for reducing the beam hardening effect are needed.
Figures and Tables
Fig. 1
CT lymphography. (A) Upper endoscopy for peritumoral injection of iopamidol at the CT scanning table. (B) Successful preitumoral submucosal injection of iopamidol showing swelling of the lesion. (C) Axial CT image after iopamidol injection (long arrow: SLN, Short arrow: draining lymphatic). SLN, Sentinel lymph node.
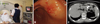
Fig. 2
Intraoperative and ex vivo SLN detection. (A) Three-dimensional volume rendering image of CT lymphography. Contrast enhanced SLN (long arrow) and draining lymphatics (short arrow) well-visualized at station #6. Dense iopamidol uptake at the peritumoral area is seen (arrow head) with a related beam hardening artifact. (B) Intraoperative SLN navigation with surserosal injection of ICG. A green colored SLN (long arrow) and a draining lymphatic (short arrow) are well identified with subserosal site injection (arrow head). (C) Ex vivo sentinel lymph node navigation using subserosal injection of ICG. SLN with ICG uptake (long arrow) and peritumoral area (arrow head) are stained green. (D) X-ray mammography. Magnified view shows the draining lymphatic vessel (short arrow) by peritumoral iopamidol injection (arrowhead). SLN, Short arrow: draining lymphatic). SLN, Sentinel lymph node; ICG, Indoc yanine green.

Fig. 3
Results of CT lymphography with iopamidol according to the location of the primary lesion. Success refers to the primary lesion that SLNs were seen on CT lymphography. Failure refers to the primary lesion that SLNs were not seen on CT lymphography. *indicates primary lesions with ulcers. SLN, Sentinel lymph node.
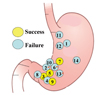
Table 1
Clinicopathologic Characteristics of the Enrolled Patients
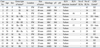
pT, pathological depth of tumor invasion, pN, pathological nodal status; SLN, sentinel lymph node; RLN, retrieved lymph node; LND: lymph node dissection; F, female; M, male; U, upper third; M, middle third; L, lower third; PW, posterior wall; LC, lesser curvature; GC, greater curvature; SRC, signet ring cell carcinoma; AWD, adenocarcinoma well-differentiated; AMD, adenocarcinoma moderately-differentiated; APD, adenocarcinoma poorly-differentiated; M, mucosa; SM, submucosa; PM, proper muscle.
*Based on Japanese Classification of Gastric Carcinoma Classification.17
References
1. Noh SH, Hyung WJ, Cheong JH. Minimally invasive treatment for gastric cancer: approaches and selection process. J Surg Oncol. 2005. 90:188–193.

2. Kitano S, Shiraishi N. Minimally invasive surgery for gastric tumors. Surg Clin North Am. 2005. 85:151–164.
3. Sano T, Katai H, Sasako M, Maruyama K. The management of early gastric cancer. Surg Oncol. 2000. 9:17–22.

4. Hiki N, Kaminishi M. Pylorus-preserving gastrectomy in gastric cancer surgery--open and laparoscopic approaches. Langenbecks Arch Surg. 2005. 390:442–447.

5. Otani Y, Furukawa T, Kitagawa Y, Yoshida M, Saikawa Y, Kubota T, et al. New method of laparoscopy-assisted function-preserving surgery for early gastric cancer: vagus-sparing segmental gastrectomy under sentinel node navigation. J Am Coll Surg. 2004. 198:1026–1031.

6. Ishikawa K, Arita T, Ninomiya S, Bandoh T, Shiraishi N, Kitano S. Outcome of segmental gastrectomy versus distal gastrectomy for early gastric cancer. World J Surg. 2007. 31:2204–2207.
7. Park do J, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008. 32:1029–1036.

8. Katai H, Sano T, Fukagawa T, Shinohara H, Sasako M. Prospective study of proximal gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2003. 90:850–853.

9. Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002. 131:1 Suppl. S306–S311.

10. Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008. 248:721–727.

11. Kunisaki C, Shimada H, Nomura M, Akiyama H. Appropriate lymph node dissection for early gastric cancer based on lymph node metastases. Surgery. 2001. 129:153–157.
12. Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004. 85:181–185.
13. Aikou T, Higashi H, Natsugoe S, Hokita S, Baba M, Tako S. Can sentinel node navigation surgery reduce the extent of lymph node dissection in gastric cancer? Ann Surg Oncol. 2001. 8:9 Suppl. 90S–93S.
14. Kitagawa Y, Fujii H, Mukai M, Kubo A, Kitajima M. Current status and future prospects of sentinel node navigational surgery for gastrointestinal cancers. Ann Surg Oncol. 2004. 11:3 Suppl. 242S–244S.
15. Bembenek A, Gretschel S, Schlag PM. Sentinel lymph node biopsy for gastrointestinal cancers. J Surg Oncol. 2007. 96:342–352.
16. Suga K, Yuan Y, Ueda K, Kaneda Y, Kawakami Y, Zaki M, et al. Computed tomography lymphography with intrapulmonary injection of iopamidol for sentinel lymph node localization. Invest Radiol. 2004. 39:313–324.

17. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998. 1:10–24.
18. Kim MC, Kim HH, Jung GJ, Lee JH, Choi SR, Kang DY, et al. Lymphatic mapping and sentinel node biopsy using 99mTc tin colloid in gastric cancer. Ann Surg. 2004. 239:383–387.

19. Lee JH, Ryu KW, Kim CG, Kim SK, Lee JS, Kook MC, et al. Sentinel node biopsy using dye and isotope double tracers in early gastric cancer. Ann Surg Oncol. 2006. 13:1168–1174.

20. Lee JH, Ryu KW, Kook MC, Lee JY, Kim CG, Choi IJ, et al. Feasibility of laparoscopic sentinel basin dissection for limited resection in early gastric cancer. J Surg Oncol. 2008. 98:331–335.

21. Hayashi H, Tangoku A, Suga K, Shimizu K, Ueda K, Yoshino S, et al. CT lymphography-navigated sentinel lymph node biopsy in patients with superficial esophageal cancer. Surgery. 2006. 139:224–235.
22. Suga K, Shimizu K, Kawakami Y, Tangoku A, Zaki M, Matsunaga N, et al. Lymphatic drainage from esophagogastric tract: feasibility of endoscopic CT lymphography for direct visualization of pathways. Radiology. 2005. 237:952–960.

23. Tangoku A, Yamamoto S, Suga K, Ueda K, Nagashima Y, Hida M, et al. Sentinel lymph node biopsy using computed tomography-lymphography in patients with breast cancer. Surgery. 2004. 135:258–265.
24. Amersi F, Morton DL. The role of sentinel lymph node biopsy in the management of melanoma. Adv Surg. 2007. 41:241–256.

25. Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H. Can sentinel node biopsy indicate rational extent of lymphadenectomy in gastric cancer surgery? Fundamental and new information on lymph-node dissection. Langenbecks Arch Surg. 1999. 384:149–157.
26. Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005. 23:4490–4498.

27. Abe N, Mori T, Takeuchi H, Yoshida T, Ohki A, Ueki H, et al. Laparoscopic lymph node dissection after endoscopic submucosal dissection: a novel and minimally invasive approach to treating early-stage gastric cancer. Am J Surg. 2005. 190:496–503.

28. Wisner ER, Katzberg RW, Griffey SM, Drake CM, Haley PJ, Vessey AR. Indirect computed tomography lymphography using iodinated nanoparticles: time and dose response in normal canine lymph nodes. Acad Radiol. 1995. 2:985–993.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download