Abstract
Purpose
The purpose of this study is to provide better understanding as to how the "double" vascular arcades, in contrast to other intestinal marginal vessels, develop along the right margin of the pancreatic head.
Materials and Methods
In human fetuses between 8-30 weeks, we described the topographical anatomy of the vessels, bile duct, duodenum as well as the ventral and dorsal primordia of the pancreatic head with an aid of pancreatic polypeptide immunohisto-chemistry.
Results
The contents of the hepatoduodenal ligament crossed the superior side of the pylorus. Moreover, the right hepatic artery originating from the superior mesenteric artery ran along the superior aspect of the pancreatic head. An arterial arcade, corresponding to the posterior pancreaticoduodenal arteries, encircled the superior part of the pancreatic head, whereas another arcade, corresponding to the anterior pancreaticoduodenal arteries, surrounded the inferior part. The dorsal promordium of the pancreas surrounded and/or mixed the ventral primordium at 13-16 weeks. Thus, both arterial arcades were likely to attach to the dorsal primordium.
Conclusion
The fetal anatomy of the pancreaticoduodenal vascular arcades as well as that of the hepatoduodenal ligament were quite different from adults in topographical relations. Thus, in the stage later than 30 weeks, further rotation of the duodenum along a horizontal axis seemed to be required to move the pylorus posterosuperiorly and to reflect the superior surface of the pancreatic head posteriorly. However, to change the topographical anatomy of the superior and inferior arterial arcades into the final position, re-arrangement of the pancreatic parenchyma might be necessary in the head.
The basic arterial configuration of the pancreaticoduodenal region is comprised of a series of the marginal vessels; i.e., anterior superior pancreaticoduodenal artery (ASPDA), anterior inferior pancreaticoduodenal artery (AIPDA), posterior superior pancreaticoduodenal artery (PSPDA) and posterior inferior pancreaticoduodenal artery (PIPDA) and their vasa recta.1-3 However, to the best of our knowledge, little embryological discussion had been performed as to why the double arcades exist there: do the different arteries supply the dorsal and ventral primordia of the pancreatic head simply because of the different origins? Actually, Sakamoto, et al.4 considered that, in adults, the dorsal pancreas is supplied by the PSPDA and the ventral pancreas by the ASPDA according to their dissection and immunohistochemistry of adult cadavers. Identification of the dorsal and ventral primordia of the pancreas, using adult as well as fetal materials, had been conducted with the aid of pancreatic polypeptide (PP) immunohistochemistry4-8 and the results provided a concept of segmentectomy for benign tumors in the pancreatic head.9,10 However, in contrast to the detailed studies of the pancreatic duct system,11,12 the arterial configuration was not investigated in the context of the pancreatic primordia or segments except for Sakamoto, et al.4 possibly because of no basic embryologic study on arterial development of the pancreatic head. Consequently, in combination with studies using PP immunohistochemistry, the present study was aimed to describe development of the vascular topographical anatomy at and around the pancreatic head in order to provide a better understanding of the "segmental" treatment in pancreatic surgery.
According to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000), we examined 20 fetuses of early and late stages: 9 fetuses between 8 and 16 weeks of gestation [6 males and 3 females; crown rump length (CRL), 35-110 mm] and 11 fetuses between 20 and 30 weeks (6 males and 5 females, CRL 160-280 mm). With agreement of the families, the ten specimens earlier than 18 weeks were donated to the Department of Anatomy, Chonbuk National University in Korea, and the use of the fetuses for research was approved by the university ethics committee. The fetuses were obtained by induced abortions. After the abortion, the mother was orally informed by an obstetrician (a single hospital) about donation for research: thus, she had never been encouraged for the donation. If the mother agreed, the fetuses were stocked in 10% w/v formalin solution with a specimen number during more than 3 months. Because of the randomized number, we were not able to find the family even if attempted. In contrast, the late staged group had been fixed with immersion in 10% formalin solution and preserved as a collection of the Medical Museum of Sapporo Medical University in Japan for medical education and research for more than 30 years. Because there was no possibility to contact persons concerned and because of collection of the Museum, the project using late stage materials did not include a specific protocol that was examined and approved by a suitably constituted institutional ethics committee.
The early staged group was decalcified using ethylenediaminetetraacetic acid (EDTA) (pH 7.5, 0.5 mol/L; Decalcifying solution B, Wako, Tokyo, Japan), whereas, because of advanced calcification, the late staged group was decalcified using Plank-Rychlo solution (AlCl2-6H2O 7.0% w/v, HCl 3.6% w/v, HCOOH 4.6% w/v; Decalcifying solution A, Wako, Tokyo, Japan). After decalcification, all specimens were prepared for paraffin embedded histology. Thus, the late staged group was inadequate for immunohistochemistry. The paraffin sections examined were 5 microns thick (early staged materials) or 10 microns thick (late staged materials) and had been cut serially (early stage) or at 100 micron intervals (late stage). Horizontal or sagittal sections were prepared: 5 for horizontal and 4 for sagittal sections in the early staged fetuses, while 7 for horizontal and 4 for sagittal sections in the late stage fetuses. Most of these sections were stained with hematoxylin and eosin (HE staining), while some of the sagittal sections of the early stage (4 fetuses; 13-16 weeks) were used for PP immunohistochemistry without pretreatment by microwave. The primary antibody used was anti-human PP (rabbit polyclonal; Yanaihara Institute Inc., Fujinomiya, Japan). Consequently, the number of sections were more than 100 (or 200) per an early (or a late) staged fetus.
At 8-12 weeks, the dorsal mesogastrium, containing the dorsal pancreas, was separated from the left adrenal cortex and kidney by the primitive peritoneal cavity (Fig. 1). Figs. 1C and D display a typical morphology of a complex of the dorsal mesogastrium, hepatoduodenal ligament, mesoduodenum and mesentery for the jejunum and ileum. The complex of mesenteries provided a thick peritoneal fold that extended along the midsagittal line and contained branches of the superior mesenteric artery (SMA) and celiac trunk as well as the pancreatic head. Michels13 termed it the "mesenteric trunk". Although the distance between the vascular branches and course changed during later development, the branching pattern was fixed in this early stage (Fig. 1): the common hepatic artery issues the gastroduodenal artery and the latter after giving off the PSPDA, divides into the ASPDA and right gastro-epiploic artery. Other branches of the celiac trunk (i.e., the splenic artery, gastroduodenal artery, PSPDA and ASPDA), and even the thin right gastric artery, were able to be identified in 8 weeks. The PSPDA was traceable along the course toward the common bile duct. Branches of the SMA, such as the PIPDA, AIPDA and jejunal arteries, were identified in the inferior side of the major duodenal papilla (not shown in Fig. 1). The PSPDA was seen in the superior side of the ASPDA.
At 13-16 weeks, observations were concentrated to the immunohistochemistry. The PP immunoreactive area was located along the common bile duct and/or in the posterosuperior part of the pancreatic head (Fig. 2). The area was situated in the posterior side of the inferior courses of the superior mesenteric vein (SMV) and portal vein (Fig. 2B). However, the area did not reach the inferior end of the head. Moreover, the gastroduodenal artery and proximal course of the ASPDA did not attach to the PP immunoreactive cells (Figs. 2B and D). In 2 of the 4 fetuses with PP immunohistochemistry, the negative immunoreactive parenchyma protruded inferiorly along the anterior aspect of the positive area (Fig. 2E). Thus, in the pancreatic head of the 2 fetuses, the PP immunoreactive area appeared to be completely surrounded by the negative parenchyma. Notably, the hepatoduodenal ligament ran anterosuperiorly in the superior side of the pylorus and the first portion of the duodenum (Fig. 2E).
In fetuses of 20-30 weeks, the portal vein and SMV divided the superior part of the pancreatic head into the anterior and posterior masses. The anterior mass continued to the pancreatic body extending leftward and superiorly, whereas the posterior mass extended inferiorly with or without tilting to the left side of the body (Figs. 3 and 4). The anterior mass of the pancreatic head attached to the first portion of the duodenum, whereas the posterior mass to the second and third portions. The AIPDA and PIPDA were clearly identified in this stage (Figs. 5 and 6). A neck or narrow part of the parenchyma was seen between the anterior and posterior masses in horizontal sections (Figs. 5B and 6B). The anterior mass was consistently located in the anterior side of the SMA, while the posterior mass sometimes (in 3 of 11 late staged fetuses) extended inferomedially to the posterior side of the SMA (Fig. 6D). The common bile duct and major duodenal papilla was involved in the posterior mass of the pancreatic head. Notably, the hepatodudenal ligament was located in the superior side of the pylorus (Figs. 3 and 4). By a connective tissue band, the fourth and/or third portions were connected to a distinct fascia in front of the left renal vein and right adrenal cortex (Fig. 6).
In 20-30 weeks, all major arteries were associated with nerve fiber bundles. In particular, the SMA was tightly surrounded by the nerve plexus. In contrast, the portal vein and its tributaries were "naked" in the loose connective tissue (Figs. 5 and 6). The gastroduodenal artery exhibited a straight course running inferiorly: its course was clearly identified in sagittal sections (Fig. 2B). The PSPDA ran along a septum-like fascia that separated a thin superficial parenchyma from the major parts of the head (Fig. 5C). In contrast, the ASPDA ran around the inferiormost part of the pancreatic head and, usually, it was not embedded in the parenchyma but separated from the parenchyma by a loose connective tissue. Thus, the ASPDA and PSPDA were not seen in a single horizontal section but separated clearly: a distance was measured as 3-6 mm between the origins of the PSPDA and ASPDA. The AIPDA and PIPDA were thin but identifiable. They displayed a tortuous course and either or both of them originated from the upper jejunal artery. Henle's gastrocolic trunk and its tributaries (the middle colic vein and right gastro-epiploic vein) ran horizontally in the anterior side of the inferiormost part of the pancreatic head (Figs. 5E and 6D).
The celiac trunk origin was located in the immediately superior side of the origin of the SMA in both of the early and late stages of fetuses: 0.1-0.2 mm in 8-13 weeks and around 2-4 mm in 20-30 weeks. At 20-30 weeks, the anterior mass of the pancreatic head extended superiorly 0.5-1.0 mm beyond the splenic arterial origin. The left-right width of the pancreatic head in levels including the major papilla reached 4-8 mm. This was usually smaller than the height (craniocaudal length) of the head (Figs. 5 and 6). The length of each portion of the duodenum was different between specimens: some fetuses carried the long first portion (Fig. 6), while the other had the short (Fig. 5).
In 4 of the 11 late staged fetuses, the right hepatic artery coexisted with the usual proper hepatic artery (Figs. 3 and 4). The right hepatic artery, at its origin, consistently issued the PIPDA and sometimes issued the inferior pancreatic artery (Fig. 5). Notably, the artery did not run along the posterior side of the pancreatic head but along the superior side. Moreover, being similar to the portal vein and SMV (Figs. 2, 3 and 4), it ran between the anterior and posterior masses of the pancreatic head.
The topographical relation between the PSPDA and ASPDA remained unchanged: the former was located superiorly, whereas the latter inferiorly (not posteriorly and anteriorly). A critical difference between stages was found in the topographical anatomy of the duodenum: 1) at 8 weeks, the third portion of the duodenum was located far inferior to the pancreatic head; 2) at 15 weeks, the third portion was slightly inferior to the first portion (Fig. 2C); 3) at 20-30 weeks, it attached to the posterior side of the pancreatic head (Figs. 5E and 6C). Thus, in the late staged group, the second portion ran posteriorly rather than inferiorly, while the third portion, in the immediately anterior side of the left renal vein, occupied in the almost same craniocaudal level as the second portion and pylorus (Fig. 6C) or in the slightly inferior level (Fig. 5E).
The present study first demonstrated the fetal topographical anatomy of the artery and vein at and around the pancreatic head: the observations seemed to reach the level beyond previous reports.14,15 The PSPDA-PIPDA (or the ASPDA-AIPDA) was likely to correspond to the superior (or the inferior) arterial arcade of the fetal pancreatic head, especially of the posterior mass of the head (Fig. 7), although the mass seemed to be composed of a mixture of the dorsal and ventral primordia. It seemed to be reasonable that the fetal pancreatic head with a relatively great "height" accompanies the two arterial arcades. Michels13 described that two primitive pancreaticoduodenal arterial arcades are located respectively on the right and left sides of the fetal pancreatic head. However, this topographical anatomy was different from the present observation: both ran along the right aspect facing the second portion of the duodenum. Another striking observation was found in the topographical anatomy of the hepatoduodenal ligament: the proper hepatic artery, portal vein and common bile duct crossed the superior aspect of the pylorus and first portion of the duodenum. Moreover, in contrast to the posterior course in adults, the right hepatic artery of the SMA origin crossed the superior aspect of the pancreatic head. Therefore, far beyond suggestions from previous reports, the fetal topographical anatomy was quite different from adults at and around the pancrteatic head. Table 1 summarizes changes required for developments later than 30 weeks to provide the adult topographical anatomy. A well-known counterclockwise rotation of the intestine along the SMA is unlikely to change the topographical relations.13,16 Which mechanism does cause the changes of the topographical relations?
We hypothesized that a simple rotation along the horizontal axis through the third portion of the duodenum moves the contents of the hepatoduodenal ligament into the posterior side of the pylorus (Fig. 7). The driving force for the rotation might be generated by a rapid growth of the stomach and right liver in the later stage in comparison with a slowdown of growth of the left liver and Spiegel's lobe.17 A sudden and great reduction in size of the right adrenal cortex after birth was also likely to cause a postero-inferior rotation of the right liver. Thus, the right liver should "fall" into a large posterior space along the body wall and diaphragm. A tight fascial connection between the duodenum and another fascia along the left renal vein, possibly corresponding to a primordum of the so-called Treitz' ligament, seemed to support the duodenum and to maintain the rotation axis. According to Kanagasuntheram,18 the intimate relation was already established at around 10 weeks of gestation (CRL 45 mm) between the left renal vein and third portion of the duodenum. As a result of the hypothetical rotation, the almost horizontally extending second portion would become perpendicular along the craniocaudal axis. Because the fetal PSPDA and ASPDA were already located on the right side of the portal and superior mesenteric veins, the left-right orientation should be maintained during the rotation.
Nevertheless, to change the topographical anatomy of the fetal superior arterial arcade into the final posterior position, "another" mechanism, such as reposition of parenchyma in the pancreatic head, seemed to be necessary. Without an assumption that the inferior arterial arcade becomes an anterior arcade due to reposition of the inferior parenchyma, the transformation process seemed not to be explicable. We hypothesized that 1) bending and mixing of the inferior parenchyma and/or 2) lower growth rate in the inferior parenchyma than other parts of the pancreatic head. Likewise, Michels13 reported a "partial" rotation of the head due to unequal growth of the duodenal wall. Actually, the present immunohistochemistry revealed that, at 13-16 weeks, the ventral primordium of the pancreas was covered by the dorsal primordium. This observation suggested joining the processes between the ventral and dorsal primordial: it was not a simple fusion. The PSPDA often ran along a thin septum between the superficial parenchyma and other major parts of the pancreatic head in the late stage fetuses. This observation seemed to correspond to a process of the dorsal primordial covering around the ventral primordium. Fig. 7 includes these hypothetical joining processes with an assumption that the anterior and posterior masses of the pancreatic head, typically seen at 20-30 weeks, primarily corresponded to the dorsal or ventral primordium, respectively.
Because of complicated processes of mixing between the ventral and dorsal primordia, fusion planes between them seemed not to be simple and flat but irregularly curved and varried between individuals. This was consistent with significant interindividual variations of the duct system in the pancreatic head.11,12 However, surgeons might expect a clear frontal plane even based on PP immunohistochemistry of adult materials.4,7,8 In contrast to the ASPDA whose origin was embedded in the dorsal primordium, the developing PSPDA displayed an intimate topographical relation with the common bile duct. This developmental process was consistent with the fact that, in adults, the major arterial supply of the common bile duct is the PSPDA.19,20 The present result of immunohistochemistry was different from the previous studies by anatomists and pathologists using fetuses and neonates: the inferior part in Paulin and Dubois5 and the postero-inferior part in Rahier, et al.6 Indeed, because the gastroduodenal artery is attached to the dorsal primordium, the ventral primordium seemed not to be located in the superior part of the "adult" pancreatic head. However, the results should be described according to orientation in the materials.
Figures and Tables
Fig. 1
Horizontal sections showing early development of the arteries around the pancreatic head (9 weeks of gestation). (A) corresponds to the superiormost of the figure. Intervals between panels are 0.6 mm (A-B), 0.1 mm (B-C), 0.2 mm (C-D), 0.2 mm (D-E), 0.15 mm (E-F), 0.05 mm (F-G) and 0.1 mm (G-H), respectively. (A) displays the origin of the celiac trunk (CT). The left gastric artery (LGA) reached the stomach (S) in the same section because of the tortuous course. (B) 0.1 mm inferior to the origin of the superior mesenteric artery (SMA), the CT was already divided into the common hepatic artery (CHA) and splenic artery (SPA). (C) contains longitudinal sections of the right gastric artery (RGA) and common hepatic artery (CHA). Between panels B and C, the hepatoduodenal ligament reaches the liver hilar region. A thick bile duct in the liver hilar region (arrow in panel C) may be the primordium of the gall bladder. (D) exhibits the PSPDA and the gastroduodenal artery (GDA) in the anterior side of the pancreatic head (star). (E) suggests that the ventral and dorsal primordia of the pancreas were already fused. A wall of the portal vein (asterisk) was destroyed. (F, G and H) contains the right gastro-epiploic artery (RGEA) and ASPDA. (G) exhibits the minor duodenal papilla (minor p). In panels F and G, the splenic vein (SPV) is cut longitudinally and, in the immediately posterior side of the SMA, a tissue (asterisk) is lost in the histological procedure. The dorsal mesogastrium (black or white arrows in panel H) containing the pancreatic body (PB) is separated from the mesentery of the midgut containing the pancreatic head (star) by a narrow peritoneal space. All sections are of the same magnification (scale bar is shown in panel H). AG, adrenal gland (cortex); AO, aorta; BV, body of the vertebrae; CBD, common bile duct; CL, caudate lobe; D1 or D2, first or second portion of the duodenum; G, gonad and mesonephros; K, kidney; NC, prevertebral condensation of the neural crest-derived cells; PHA, proper hepatic artery; PY, pylorus; S3, segment III of the liver; SMV, superior mesenteric vein; SP, spleen; UV, umbilical vein.
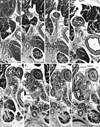
Fig. 2
Sagittal sections of the pancreatic head with pancreatic polypeptide (PP) immunohistochemistry (16 weeks of gestation). The left-hand side of the figures corresponds to the posterior side of the body. (A-E) displays the same fetus with 16 weeks of gestation. The PP immunoreactive areas are colored yellow in panels A-C, while the positive cells (brown-colored) are demonstrated in (D) and (E). (D), a near section of panel B, corresponds to the central part of (B). Interval between panels are 0.2 mm (A-B) and 0.6 mm (B-C), respectively (panel A is the right side of panels B and C). The PP immunoreactive areas are located along the common bile duct, in the posterosuperior part of the pancreatic head (Panc) and/or in the posterior side of the superior mesenteric vein (SMV). However, the area does not reach the inferior end of the head. The gastroduodenal artery and proximal course of the ASPDA do not attach to the PP immunoreactive cells (panels B, D and E). In (E) the negative immunoreactive parenchyma protruded inferiorly along the posterior aspect of the positive area. Note that the hepatoduodenal ligament (HD-lig) riding over the first portion of the duodenum (D1). D3, the third portion of the duodenum; Ggl, celiac ganglion; LF, lymph follicle; NPX, nerve plexus; PV, portal vein; RPV, retropancreatic vein; TC, transverse colon. Other abbreviations are the same as in Fig. 1. The magnification of panels A-C is different from (D) and (E) [scale bars, (B) and (E)].
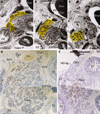
Fig. 3
A diagram showing arteries at and around the pancreatic head of a female fetus with 23 weeks of gestation. This diagram is based on observations of more than 200 horizontal sections of a fetus, parts of which will be shown in Fig. 5. The pancreas is composed of a transversely extending anterior mass (labeled by dots; almost corresponding to the dorsal pancreas) and a standing-up posterior mass of the pancreatic head (labeled by clusters of #; almost corresponding to the ventral pancreas). The hepatic artery (HA), portal vein (PV) and common bile duct (CBD) go superiorly and rightward across the superior side of the first portion of the duodenum. The right hepatic artery (RHA) originates from the superior mesenteric artery (SMA), issues the PIPDA and runs along the superior surface of the pancreatic head. The gastroduodenal artery (GDA) displayed a downward course. The PSPDA-PIPDA encircles the superior part of the pancreatic head, whereas the ASPDA-AIPDA runs around the inferiormost part. The inferior mesenteric vein (IMV) crosses the posterior aspect of the fourth portion of the duodenum. Because of a limitation of the drawing skill, the portal vein is separated from the pancreatic head in the inferior side of the figure. Other abbreviations are the same as in Fig. 1.
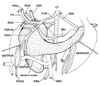
Fig. 4
A diagram showing arteries at and around the pancreatic head of a female fetus with 25 weeks of gestation. This diagram is based on observations of more than 200 horizontal sections of a fetus, parts of which will be shown in Fig. 6. In comparison with the diagram shown in Fig. 3, a transversely extending anterior mass of the pancreas (labeled by dots; almost corresponding to the dorsal pancreas) is slender while an obliquely positioned posterior mass [labeled by sharps (#); almost corresponding to the ventral pancreas] is thick. Arteries at and around the pancreatic head display courses similar to Fig. 3. The right hepatic artery (RHA) originates from the superior mesenteric artery (SMA) and issues the PIPDA and inferior pancreatic artery (IPA). The inferior mesenteric vein (IMV) crosses the anterior aspect of the fourth portion of the duodenum. Other abbreviations are the same as in Figs. 1 and 3.
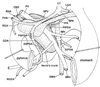
Fig. 5
Horizontal sections at and around the pancreatic head of a female fetus with the same 23 weeks-fetus as shown in Fig. 3. (A) corresponds to the superiormost of the figure. Intervals between panels are 2.2 mm (A-B), 1.8 mm (B-C), 2.0 mm (C-D), 1.1 mm (D-E), and 1.2 mm (E-F), respectively. (A) displays the splenic, common hepatic and superior mesenteric arteries near their origins (SPA, CHA, SMA). (B) exhibits the origin of the right hepatic artery (RHA) from the SMA. The gastroduodenal artery (GDA) and PSPDA are cut transversely in the anterior aspect of the pancreatic head (star). (C) shows origins of the right gastro-epiploic artery (RGEA) and ASPDA. The PSPDA is cut longitudinally and embedded in the superficial parenchyma of the pancreatic head (star). (D) displays the major and minor duodenal papillae (major p, minor p). Note that these two papillae are seen in a single horizontal section, not in a sagittal or frontal section. (E) contains the middle colic vein (MCV) and right gastroepiploic vein (RGEV) (i.e., Henle's gastrocolic venous trunk) and upper jejunal arteries (JA). (F) exhibits the ASPDA-AIPDA running around the inferiormost part of the pancreatic head (star). The SMA is surrounded by a thick nerve plexus (panels B-F). D1, D2, D3 and D4, the first-fourth portions of the duodenum; IVC, inferior vena cava; LF, lymph follicle; LRV, left renal vein; PM, psoas major; TC, transverse colon. Other abbreviations are the same as in Fig. 1.
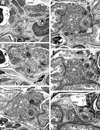
Fig. 6
Horizontal sections at and around the pancreatic head of a female fetus with the same 25 weeks-fetus as shown in Fig. 4. (A) corresponds to the superiormost of the figure. Intervals between panels are 0.9 mm (A-B), 2.0 mm (B-C) and 3.0 mm (C-D), respectively. (A) in the 6 mm inferior to the origin of the celiac trunk, displays the inferior pancreatic artery (IPA) arising from the right hepatic artery (RHA). (B) exhibits the origin of the RHA from the SMA. From the origin, the PIPDA is issued to the posterior side of the pancreatic head. In (B), the inferior mesenteric vein (IMV) is cut longitudinally and drains into the portal vein (PV). The fourth portion of the duodenum (D4) is connected by a tight connective tissue (arrow in panels A and B) to fasciae covering the left renal vein (LRV). (C) contains the ASPDA and right gastro-epiploic artery (RGEA). The upper jejunal artery (JA) issues the AIPDA. (D) displays the longitudinal sections of the ASPDA-AIPDA and Henle's venous trunk (the middle colic vein or MCV and the right gastro-epiploic vein or RGEV). Parts of the pancreatic head (star) are located in the posterior side of the superior mesenteric artery and vein because the vessels run transversely rather than inferiorly. LF, lymph follicle. Other abbreviations are the same as in Fig. 1.
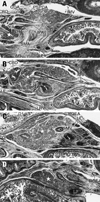
Fig. 7
A diagram showing joining processes between the dorsal and ventral primordia of the pancreas as well as the hypothetical rotation of the duodenum along a left-right axis. Viewed from the posterosuperior side of the body. A horizontal plane including most parts of the duodenum is shown to emphasize, in contrast to adults, the course of the second portion (D2) directing posteriorly rather than inferiorly. The topographical anatomy of the duodenum and pancreatic head is based on observations of fetuses of 20-30 weeks (e.g., Figs. 3, 4, 5 and 6). The anterior mass of the pancreatic head (labeled by dots) seems to correspond to the dorsal primordium of the pancreas, while an oblique or perpendicular posterior mass [labeled by sharps (#)] seems to primarily correspond to the ventral primordium. The portal vein and right hepatic artery run in the superior sides of and along their border between the anterior and posterior masses. Notably, tongue-like protrusions of the anterior mass or dorsal pancreas (stars) surround the primary posterior mass or the ventral pancreas. In particular, the right anterior protrusion (open star) often reaches the inferior end of the ventral pancreas. The common bile duct crosses the superior side of the first portion of the duodenum. We hypothesize a rotation along a left-right axis through the third portion of the duodenum in the later stage of development. In addition, the fourth portion is connected by a fascia (asterisk) to a thick fascia along the left renal vein.
ACKNOWLEDGEMENTS
This work was supported by the grant of the Post-Doc. Program, Chonbuk National University (2007) and grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620220-1).
We are grateful to Ms. Masako Kuronuma at Yamagata University for technical assistance.
References
1. Bertelli E, Di Gregorio F, Bertelli L, Mosca S. The arterial blood supply of the pancreas: a review. I. The superior pancreaticoduodenal and the anterior superior pancreaticoduodenal arteries. An anatomical and radiological study. Surg Radiol Anat. 1995. 17:97–106. 1–3.
2. Bertelli E, Di Gregorio F, Bertelli L, Civeli L, Mosca S. The arterial blood supply of the pancreas: a review. II. The posterior superior pancreaticoduodenal artery. An anatomical and radiological study. Surg Radiol Anat. 1996. 18:1–9.
3. Murakami G, Hirata K, Takamuro T, Mukaiya M, Hata F, Kitagawa S. Vascular anatomy of the pancreaticoduodenal region: a review. J Hepatobiliary Pancreat Surg. 1999. 6:55–68.

4. Sakamoto Y, Nagai M, Tanaka N, Nobori M, Tsukamoto T, Nokubi M, et al. Anatomical segmentectomy of the head of the pancreas along the embryological fusion plane: a feasible procedure? Surgery. 2000. 128:822–831.
5. Paulin C, Dubois PM. Immunohistochemical identification and localization of pancreatic polypeptide cells in the pancreas and gastrointestinal tract of the human fetus and adult man. Cell Tissue Res. 1978. 188:251–257.

6. Rahier J, Wallon J, Gepts W, Haot J. Localization of pancreatic polypeptide cells in a limited lobe of the human neonate pancreas: remnant of the ventral primordium? Cell Tissue Res. 1979. 200:359–366.

7. Tadokoro H, Kozu T, Toki F, Kobayashi M, Hayashi N. Embryological fusion between the ducts of the ventral and dorsal primordia of the pancreas occurs in two manners. Pancreas. 1997. 14:407–414.

8. Uchida T, Takada T, Ammori BJ, Suda K, Takahashi T. Three-dimensional reconstruction of the ventral and dorsal pancreas: a new insight into anatomy and embryonic development. J Hepatobiliary Pancreat Surg. 1999. 6:176–180.
9. Takada T. Vantral pancreatectomy: resection of the ventral segment of the pancreas. J Hepatobiliary Pancreat Surg. 1993. 1:36–40.
10. Ryu M, Takayama W, Watanabe K, Honda I, Yamamoto H, Arai Y. Ventral pancreatic resection for adenoma and low-grade malignancies of the head of the pancreas. Surg Today. 1996. 26:476–481.
11. Takahashi S, Akita K, Goseki N, Sato T. Spatial arrangement of the pancreatic ducts in the head of the pancreas with special reference to the branches of the uncinate process. Surgery. 1999. 125:178–185.
12. Kamisawa T, Egawa N, Tu Y, Tsuruta K, Okamoto A. Pancreatographic investigation of embryology of complete and incomplete pancreas divisum. Pancreas. 2007. 34:96–102.
13. Michels NA. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. 1955. Philadelphia: Lippincott;24–133.
14. Adda G, Hannoun L, Loygue J. Development of the human pancreas: variations and pathology. A tentative classification. Anat Clin. 1984. 5:275–283.
15. Krakowiak-Sarnowska E, Flisiñski P, Szpinda M, Flisiński M, Sarnowski J. The pancreaticodudenal arteries in human foetal development. Folia Morphol (Warsz). 2004. 63:281–284.
16. Skandalakis JE, Gray SW. Embryology for surgeons: the embryological basis for the treatment of congenital anomalies. 1994. Baltimore: Williams & Wilkins;396–404.
17. Lee SD, Kim CY, Cho YH, Fujiwara D, Murakami G, Mutsumura H, et al. Morphometrical data of size and shape of the late-stage human fetal liver, including those of intrahepatic vessels: some prenatal and postnatal developmental consideration. Korean J Hepatobiliary Pancreat Surg. 2003. 7:12–18.
18. Kanagasuntheram R. Some observations on the development of the human duodenum. J Anat. 1960. 94:231–240.
19. Kimura W, Nagai H. Study of surgical anatomy for duodenum-preserving resection of the head of the pancreas. Ann Surg. 1995. 221:359–363.

20. Furukawa H, Iwata R, Moriyama N, Kosuge T. Blood supply to the pancreatic head, bile duct, and duodenum: evaluation by computed tomography during arteriography. Arch Surg. 1999. 134:1086–1090.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download