Abstract
Purpose
The aim of this study was to determine whether retinol-binding protein 4 (RBP4), adiponectin and high molecular weight (HMW) adiponectin are associated with insulin resistance (IR) and metabolic parameters in non-diabetic hypertensive patients. Also, we sought to compare the predictive values of these adipocytokines for IR in non-diabetic hypertensive patients.
Materials and Methods
Analyses of RBP4, adiponectin, and HMW adiponectin were performed on 308 non-diabetic hypertensives (148 males, age 58 ± 10 years, 189 non-metabolic syndrome and 119 metabolic syndrome). The homeostasis model assessment (HOMA) index for IR, lipid profiles, and anthropometric measure-ments were assessed.
Results
There was no significant difference in RBP4 levels according to the presence of metabolic syndrome, although adiponectin and HMW adiponectin were significantly lower in metabolic syndrome. Correlation analysis of log RBP4 with IR and metabolic indices revealed that there was no significant correlation of RBP4 with waist circumference (r = 0.056, p = 0.324), HDL cholesterol (r = 0.005, p = 0.934), ApoB/ApoAI ratio (r = 0.066, p = 0.270), and the HOMA index (r = 0.017, p = 0.756). However, adiponectin and HMW adiponectin showed significant correlations with the HOMA index (r = - 0.247, p < 0.001; r = - 0.296, p < 0.001) and metabolic parameters. With IR defined as HOMA index ≥ 2.5, HMW adiponectin did not demonstrate a superior predictive value for IR compared to adiponectin (AUC = 0.680 vs. 0.648, p = 0.083). The predictive value of RBP4 for IR was minimal (AUC = 0.534).
Conclusion
RBP4 was not associated with IR or metabolic indices and the predictive value for IR was minimal in hypertensives. HMW adiponectin didn't have a superior predictive value for IR compared to adiponectin. Therefore, we can suggest that RBP4 and HMW adiponectin don't have more additive information than adiponectin in non-diabetic hypertensives.
Epidemiologic studies have demonstrated that hypertension is usually accompanied by major cardiovascular risk factors with less than 20% of hypertension occurring as an isolated manifestation.1-3 The risk of cardiovascular events in hypertensive patients increases in proportion to the burden of associated risk factors.1,4 Adipocytokines may have a significant role in the manifestation of risk factors in hypertensive patients. Of the studies on adipocytokines, adiponectin is the most abundant adipocytokine in human circulation and is known to be decreased in patients with hypertension.5,6 In human cross-sectional studies, plasma adiponectin levels are negatively correlated with obesity and waist-hip ratio, diabetic dyslipidemia, insulin resistance (IR), and cardiovascular disease.7,8 Therefore, the level of plasma adiponectin may be an important determinant of associated cardiovascular risk factors in hypertension as well. Recent studies have demonstrated the possible importance of novel adipocytokines, such as retinolbinding protein 4 (RBP4) and high molecular weight (HMW) adiponectin, as a mediator for IR.9-12 However, some studies have reported contradictory findings regarding the association of RBP4 with IR.13,14 The added predictive value of HMW adiponectin compared to conventional adiponectin also needs further validation; therefore, in this study, we sought to determine the association between RBP4, adiponectin, and HMW adiponectin with IR, and metabolic cardiovascular risk factors in hypertensive subjects.
This was a cross sectional analysis of 308 non-diabetic patients with hypertension. All subjects were enrolled in the Seoul Metabolic Syndrome Research Initiatives, a study sponsored by the city of Seoul to develop a prospective cohort for hypertension and metabolic syndrome. We enrolled non-diabetic treated hypertensive patients with either a documented systolic blood pressure greater than 140 mmHg and/or diastolic blood pressure greater than 90 mmHg taken over three different visits after at least 5 minutes rest in a sitting position prior to the commencement of a blood pressure medication regimen or patients currently taking antihypertensive medications for treatment of hypertension at the time of enrollment. Patients with the following conditions were excluded from the study: Those with a significant history of valvular heart disease, systemic disease and systemic inflammatory disease; congestive heart failure; and serum creatinine levels greater than 1.4 mg/dL. This study was approved by the institutional ethics committee and the procedures were followed in accordance with institutional guidelines. All of the patients gave written informed consent prior to the study.
Body weight and height measurements were taken in the morning while subjects were unclothed and without shoes. Body mass index (BMI) was calculated as body weight in kilograms per height2 (m2). Waist circumference was taken with a tape measure horizontally at the umbilicus while subjects were in the standing position after normal expiration. Blood pressure was measured from the dominant arm of seated patients with a sphygmomanometer after 5 minutes of rest in sitting position.
Metabolic syndrome was diagnosed based on the Asian-modified NCEP criteria15 if 3 or more of the following conditions were present: high blood pressure (systolic and/or diastolic blood pressures ≥ 130/85 mmHg or patients receiving anti-hypertensive drugs), hyperglycemia (fasting plasma glucose ≥ 110 mg/dL or patients receiving oral hypoglycemic agents), hypertriglyceridemia (fasting plasma triglycerides ≥ 150 mg/dL), low HDL cholesterol (fasting HDL cholesterol < 40 mg/dL for men and < 50 mg/dL for women), or central obesity (waist circumference of ≥ 90 cm for men and ≥ 80 cm for women). IR was defined as homeostasis model assessment (HOMA) index of ≥ 2.5 according to the Japanese guideline for the treatment of diabetes.12
Venous blood specimens were collected in EDTA-treated and plain tubes after a 12 h fast. The tubes were immediately placed on ice until they were transported to the laboratory (within 1-3 h) and stored at -70℃ until analysis.
Blood chemistry, including fasting glucose, blood urea nitrogen, creatinine, uric acid, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, and fasting serum insulin, were assessed. Fasting serum insulin levels were measured using an immunoradiometric assay and a gamma counter (Hewlett Packard, Meriden, Connecticut, USA). IR was measured using the homeostasis model assessment of the IR index (HOMA index) by the following formula: HOMA index = [fasting insulin (µU/mL)×fasting glycemia (mmol/L)] / 22.5.
Apolipoprotein B and apolipoprotein A assays were measured by immunoturbidimetry (Roche Diagnostics, Basel, Switzerland) as described previously.16,17
Plasma adiponectin was measured using a radioimmunoassay (LINCO, St. Charles, Missouri, USA). Plasma RBP4 was measured using ELISA (Immunodiagnostik, Bensheim, Germany). Plasma HMW adiponectin was measured using ELISA (LINCO, St. Charles, Missouri, USA). Plasma HMW adiponectin/total plasma adiponectin ratio was calculated as the ratio of HMW adiponectin/(HMW adiponectin + low molecular weight adiponectin) based upon a previous study by Pajvani, et al.18
The study population was divided according to the presence or absence of metabolic syndrome. Continuous variables are presented as mean ± standard deviation and categorical variables as absolute and relative frequencies (%). Continuous variables were compared using the student's t-test. Categorical variables were compared using chi-square test. Simple correlation analyses were performed to determine the association between RBP4, adiponectin, HMW adiponectin, variables of IR, and metabolic cardiovascular risk factors. To normalize the distribution, a natural logarithmic transformation was applied to variables that were not in the normal distribution. To determine the independent association of the adipocytokines with cardiovascular risk factors, multiple linear regression was performed with age, the HOMA index, triglyceride levels, HDL cholesterol, and the ApoB/ApoAI ratio as dependent variables. The predictive power of each adipocytokines for identifying patients with IR was analyzed using the receiver operating characteristic (ROC) curve analysis. Statistical analyses were done using SPSS statistical software package (version 13.0, SPSS Inc., Chicago, IL, USA). Comparisons of the ROC curves were performed using MedCalc statistical software package (version 9.3.3.0, Mariakerke, Belgium). A p-value of < 0.05 was considered to be statistically significant.
The age of the study population was 58 ± 10 years. There were 148 males and 160 females analyzed in this study. Among the 308 patients, 119 patients met the NCEP criteria for metabolic syndrome. There were no significant difference in blood pressure, total cholesterol, LDL cholesterol, serum creatinine, and hsCRP among the two groups but females were predominant in the group with metabolic syndrome. The metabolic parameters were significantly different between the two groups because of the study design (Table 1).
Most of the subjects enrolled in the study were well controlled hypertensive patients taking antihypertensive medications at the time of enrollment. The relatively low value of LDL cholesterol in this study population may be explained as 29.5% of the study population was taking statins at the time of enrollment (Table 1). The total cholesterol and LDL cholesterol in non-metabolic syndrome without statins were 187.6 ± 33.0 mg/dL and 108.4 ± 28.2 mg/dL and with statins were 153.8 ± 34.7 mg/dL and 78.8 ± 28.7 mg/dL. Those with metabolic syndrome without statins were 188.2 ± 35.4 mg/dL and 109.1 ± 30.9 mg/dL and with statins were 171.2 ± 40.5 mg/dL and 82.9 ± 35.1 mg/dL.
Because of gender differences in the level of adipocytokines and gender distribution in the two groups, we analyzed the adipocytokines in each gender. There was no significant difference in RBP4 levels according to the presence of metabolic syndrome in both genders, although there were significantly lower levels of adiponectin and HMW adiponctin in patients with metabolic syndrome. The HMW/total adiponectin ratio was significantly lower, especially in female patients with metabolic syndrome (Table 2).
Correlation analysis of log transformed RBP4 with IR and metabolic parameters revealed that there was no significant correlation of RBP4 with waist circumference, HDL cholesterol, ApoB/ApoAI ratio, and the HOMA index, although RBP4 was correlated with triglycerides (r = 0.246, p < 0.001) and uric acid (r = 0.322, p < 0.001). There was also no significant correlation of RBP4 with adiponectin and HMW adiponectin (Fig. 1A).
The correlation analysis for adiponectin and HMW adiponectin revealed a significant correlation with metatolic parameters (Fig. 1B, C). There was a significant correlation with adiponectin, but not HMW adiponectin with ApoB/ApoAI ratio (r = - 0.133, p = 0.026, r = - 0.106, p = 0.076). There was an excellent correlation between adiponectin and HMW adiponectin (r = 0.863, p < 0.001).
Adiponectin was independently associated with the HOMA index (β = - 0.230, p < 0.001), HDL cholesterol (β = 0.304, p < 0.001), and ApoB/ApoAI ratio (β = 0.117, p = 0.039) when controlled for BMI, lipid profiles, and age. There was no significant independent association with triglycerides in this study. A similar finding was observed for HMW adiponectin. A similar trend was observed in the HMW adiponectin/total adiponectin ratio in the HOMA index and HDL cholesterol but there was a weak correlation with marginal significance (β = 0.099 , p = 0.072) between the adiponectin ratio and ApoB/Apo AI ratio (Table 3).
The ROC curves were plotted for the predictive power of adipocytokines for IR. The results showed that the area under the curve of HMW adiponectin was slightly larger than that of adiponectin without statistical significance [0.680 (95% CI 0.611-0.748)] vs. [0.648 (95% CI 0.579-0.716), p = 0.083] (Fig. 2A), suggesting that HMW adiponectin wasn't superior for predicting IR than conventional adiponectin. Also, there was no additive predictive value of the HMW adiponectin/total adiponectin ratio for predicting IR compared to adiponectin [0.652 (95% CI 0.583-0.722)] vs. [0.648 (95% CI 0.579-0.716), p = 0.734]. When a cutoff value of 7.0 ug/ml was used, plasma adiponectin predicted the presence of IR with a sensitivity of 66.3% and specificity of 60.7%. The sensitivity and specificity of HMW adiponectin for predicting IR was 70.7% and 61.8% at a cutoff value of 1.48 ug/mL, and the HMW adiponectin/total adiponectin ratio diagnosed IR with a sensitivity of 72.1% and specificity of 60.7% at a cutoff level of 1.44. Regarding RBP4, the area under the curve was only 0.534 (95% CI 0.462-0.605) in this study (Fig. 2B).
One of the key findings in this study was that plasma RBP4 was not associated with indices of metabolic syndrome, such as waist circumference, HDL cholesterol, ApoB/ApoAI ratio, and the HOMA index. However, there was a significant correlation of RBP4 with serum triglycerides.
RBP4 has been identified as an adipocytokine that is increased in circulation in mouse models of obesity and insulin resistance.18 Furthermore, transgenic expression and administration of RBP4 into mice induced insulin resistance in this previous report.19 However, some studies have reported contradictory findings regarding the association of RBP4 with IR.13-14 So, the assessment of the importance of RBP4 is needed in diverse groups. Our findings of this study are in accordance with findings reported by Takashima, et al.19, which showed that serum RBP4 did not correlate with fasting blood glucose, HDL cholesterol, waist-hip ratio, BMI, and fasting insulin, but significantly correlated with triglycerides.
There are some possible explanations for the lack of association of RBP4 with indices of IR. First, HOMA-IR may not be an ideal measurement of insulin sensitivity, especially in subjects with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT).20 Although HOMA-IR has been shown to have a strong correlation with insulin resistance in diabetic and non-diabetic subjects, the correlation was not demonstrated in subjects with IFG or IGT. Perhaps this may partially explain the lack of correlation between RBP4 and HOMA IR in this study. However, Stefan, et al.21 has reported that RBP4 is associated with an elevation of liver fat but not visceral fat in humans. Since liver fat is associated with hepatic insulin sensitivity and HOMA IR shows good correlation with hepatic insulin sensitivity, there may be some other factors responsible for the contradictory results. Perhaps the difference in results may be attributed to a difference in ethnicity, degree of obesity, and subset of the patients analyzed. This study was performed on an Asian population with BMI and HOMA indices that are relatively low compared to studies performed on Caucasians. The study was also performed on treated hypertensives with 38.6% of patients defined as having metabolic syndrome, which may have influenced the results. Second, the lack of association of RBP4 with adiponectin, HMW adiponectin, BMI, waist-hip ratio, and HDL cholesterol may be explained by the fact that RBP4 may not be associated with visceral and intramyocellular fat. Since insulin increases the rate of depletion of vitamin A from the liver, the association of RBP4 reported in previous studies may be the consequence of hepatic IR rather than the cause.22,23 Further studies are needed to clarify the mechanism for the cross-sectional association of RBP4 with metabolic indices.
Contrary to previous reports,11,12 HMW adiponectin did not demonstrate a superior predictive value for IR compared to total adiponectin with the predictive value for IR being slightly better for HMW adiponectin (AUC = 0.680) compared to adiponectin (AUC = 0.648). HMW adiponectin demonstrated a slightly stronger independent association with the HOMA index and ApoB/ApoAI ratio compared to total adiponectin. However, the HMW adiponectin/total adiponectin ratio did not demonstrate a stronger association with the HOMA index and metabolic indices. The reason for the lack of stronger association of HMW adiponectin with IR and metabolic indices is not clear. Several studies have demonstrated the superiority of HMW adiponectin compared to total adiponectin.11,12,24 However, a recent study by Blüher, et al.25 demonstrated a lack of superiority of HMW adiponectin over total adiponectin in predicting metabolic variables at baseline and in response to exercise. A possible explanation for the discrepancy may be that the relationship between metabolic variables and HMW adiponectin may be different in diabetics and non-diabetics. Studies have shown that selective reduction of posttranslational modification of the lysine residue of adiponectin results in the lowering of HMW adiponectin in diabetes, which increases in response to insulin sensitizers.25,26 The absence of diabetics in this study population may account for the lack of superior association of HMW adiponectin with metabolic variables.
The limitations of this study need to be addressed. First, the antihypertensive and lipid-lowering medications might have partially influenced the results from the data. Statins are known to increase the levels of heme oxygenase which is regarded as the primary cellular defense mechanism against increased levels of reactive oxygen species.27 Heme oxygenase and adiponectin levels move in conjunction with animal models of obesity, hypertension, diabetes, and metabolic syndrome.28,29 It was possible that the level of adipocytokines and metabolic parameters were influenced by the medications, especially statins, but the proportions of patients taking medications were not different between the groups. Second was the difference in gender distribution between the groups. To minimize the confusion, we demonstrated and compared the level of adipocytokines according to gender in the two groups. Third, the study sample only included Asians, and it is possible that results from the present study may not be applicable to other ethnic and racial groups.
In conclusion, RBP4 was not associated with IR or metabolic indices and the predictive value for IR was minimal in hypertensive subjects. HMW adiponectin did not have a superior predictive value for IR compared to adiponectin.
Figures and Tables
 | Fig. 1Correlation of RBP4, adiponectin, and HMW adiponectin with metabolic parameters in hypertensive subjects. (A) Log RBP4, (B) Log adiponectin, (C) Log HMW adiponectin. RBP4, retinol-binding protein 4; HMW, high-molecular weight. |
 | Fig. 2ROC curves of adipocytokines for prediction of insulin resistance, defined as HOMA-IR index > 2.5 (n = 308), in hypertensive subjects. (A) Plasma adiponectin and HMW adiponectin. (B) Plasma RBP4. AUC, area under curve; ROC, receiver operating characteristics; HOMA, homeostasis model assessment; RBP4, retinol-binding protein 4. |
Table 1
Baseline Clinical Characteristics According to Presence of Metabolic Syndrome
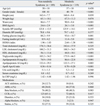
BMI, body mass index; BP, blood pressure; HOMA, homeostasis model assessment; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Apo, apolipoprotein; CRP, c-reactive protein; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
*p < 0.05 is considered significant.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A000385), and by a faculty research grant of Yonsei University College of Medicine for 2005 (6-2005-0051).
References
1. Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000. 13:3S–10S.
2. Ansell BJ. Evidence for a combined approach to the management of hypertension and dyslipidemia. Am J Hypertens. 2005. 18:1249–1257.

3. Wilson PW, Kannel WB, Silbershatz H, D'Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999. 159:1104–1109.
4. Thomas F, Rudnichi A, Bacri AM, Bean K, Guize L, Benetos A. Cardiovascular mortality in hypertensive men according to presence of associated risk factors. Hypertension. 2001. 37:1256–1261.

5. Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003. 16:72–75.
6. Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004. 43:1318–1323.

7. Baratta R, Amato S, Degano C, Farina MG, Patanè G, Vigneri R, et al. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004. 89:2665–2671.

8. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003. 46:459–469.
9. Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006. 354:2552–2563.

10. Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007. 56:327–331.

11. Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, et al. Serum high molecular weight complex of adiponectin correlates better with glucose intolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005. 48:1084–1087.

12. Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006. 29:1357–1362.

13. Janke J, Engeli S, Böhnke M, Adams F, Bhnke J, Luft FC, et al. Retinol binding protein 4 in human obesity. Diabetes. 2006. 55:2805–2810.
14. Broch M, Vendrell J, Ricart W, Richart C, Fernández-Real JM. Circulating retinol binding protein-4, insulin sensitivity, insulin secretion and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007. 30:1802–1806.
15. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004. 27:1182–1186.

16. Jungner I, Marcovina S, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998. 44:1641–1649.

17. Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I in relation to serum cholesterol and triglyceride in 43,000 Swedish males and females. Int J Clin Lab Res. 1992. 21:247–255.

18. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005. 436:356–362.

19. Takashima N, Tomoike H, Iwai N. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006. 355:1392.

20. Tripathy D, Tuomi T, Almgren P, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004. 27:2204–2210.

21. Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, et al. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007. 30:1173–1178.

23. Sasaki H, Iwasaki T, Kato S, Tada N. High retinol/retinol-binding protein ratio in non insulin-dependent diabetes mellitus. Am J Med Sci. 1995. 310:177–182.

24. Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006. 55:249–259.
25. Blüher M, Brennan AM, Kelesidis T, Kratzsch J, Fasshauer M, Kralisch S, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007. 30:280–285.
26. Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, et al. Post-translational modification of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006. 281:16391–16400.
27. Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004. 110:1296–1302.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download