Abstract
Purpose
Severe hypoglycemia can result in neural damage, impaired cognitive function, coma, seizures, or death. The decision to admit diabetic patients after initial treatment in the emergency department remains unclear. Our purpose is to identify risk factors for developing recurrent hypoglycemia in diabetic patients admitted for severe hypoglycemia.
Materials and Methods
We reviewed the records of 233 subjects (92 males, 141 females; mean age, 74.1 ± 9.8 years) with type 2 diabetes treated at a tertiary care teaching hospital and hospitalized for severe hypoglycemia.
Results
Seventy-four (31.8%) patients were categorized with recurrent hypoglycemia and 159 (68.2%) with non-recurrent. Multivariate logistic regression analysis revealed that patients with loss of a recent meal, coronary artery disease, infection, and poor renal function (lower estimated glomerular filtration rate) were at risk for recurrent hypoglycemia. The use of calcium-channel blockers appeared to be a protective factor for the development of recurrent hypoglycemia.
Hypoglycemia is a common metabolic emergency representing an imbalance in the energy demands of the brain and the available glucose supply despite normal arterial oxygen tension.1,2 This imbalance results in autonomic symptoms (e.g., anxiety, sweating, and tremulousness) and neurologic symptoms (e.g., weakness, fatigue, and mental confusion).1,3 Approximately 7% of patients presenting to the emergency department (ED) with complaints of altered mental status have hypoglycemia, and 20% of patients with diabetes mellitus (DM) treated with insulin or anti-diabetic agents (ADAs) will have symptoms related to hypoglycemia requiring ED evaluation and treatment.4 Severe hypoglycemia, if unrecognized and untreated, can result in neural damage, impaired cognitive function, coma, seizures, or death.4-7 Single and recurrent episodes of hypoglycemia may cause physical and psychological morbidity and increase the risk of death, but may also prevent euglycemia.8 Risk factors placing diabetic patients at greater risk for developing hypoglycemia include male gender, adolescence or age > 55, increased amounts of exercise or activity, decreased carbohydrate intake, alcohol consumption, impaired renal or liver function, intensive glucose control, changes in insulin or ADA regimen, infection, significant comorbidities, and polypharmacy.2,9,10
Patients with severe hypoglycemia and who are at risk for recurrent hypoglycemia should be admitted, while those with a rapidly normalized glucose level and not at risk may not require admission. The purpose of this study is to identify risk factors for recurrent hypoglycemia in diabetic patients hospitalized for severe hypoglycemia.
This retrospective, observational chart review was conducted at a 1,600-bed, tertiary-level, teaching hospital with an annual ED volume of 90,000 patients. Patients presenting to the ED during a 4-year period from January 2003 to December 2006 and diagnosed with DM and hospitalized for severe hypoglycemia were enrolled. As this study involved medical records obtained as part of routine ED care, the Institutional Review Board determined that no patient consent was required. Inclusion criteria were patients diagnosed with type 2 DM, either by previous medical records or by clinical criteria described previously,11 and followed in the diabetes clinic for at least 2 months.
We defined severe hypoglycemia using the American Diabetes Association criteria as an event requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative action. These episodes may be associated with sufficient neuroglycopenia to induce seizure or coma, and neurological recovery is attributable to the restoration of the blood glucose level.12
The hospital database is an observational database including data on all patients hospitalized at the study institution. Patient records coded for ICD9 251.× and 250.× were retrieved, and patient records not fitting the inclusion criteria were excluded. For analysis, patients were divided into 2 groups. The study group consisted of admitted diabetic patients with hypoglycemic episodes during the first 48 hours of admission. The control group consisted of admitted diabetic patients without hypoglycemic episodes after admission.
Each chart was reviewed by one of the five authors and data abstracted to a standardized collection sheet developed by the authors. Demographic data abstracted included age, gender, duration of DM (years), hospital days, body mass index (BMI), comorbidities, carbohydrate intake, loss of a recent meal (defined as a history of missing a meal recently or decreased food intake before ED visit), current medications, laboratory testing, and initial serum glucose level.
Laboratory studies abstracted were white blood count (WBC), hemoglobin, platelets, aspartate transaminase, blood urea nitrogen (BUN), serum creatinine, hemoglobin A1c (HbA1c), albumin, total cholesterol, triglyceride, total protein, and uric acid. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease study formula as follows: GFR = 186.3 × (serum creatinine-1.154)×(age-0.203)×1.212 (if black)×0.742 (if female).13
Current medications included benzodiazepines, renin-angiotensin system blockers (RASBs), calcium channel blockers (CCB), alpha-blockers, beta-blockers, diuretics, insulin, and oral ADAs. We defined polypharmacy as more than 5 of these medications.
Comorbidities included hypertension, coronary artery disease (CAD), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), dyslipidemia, infection, end-stage renal disease (ESRD), cancer, cirrhosis, and alcoholism.
The main outcome measure was the development of recurrent symptomatic hypoglycemia (serum glucose < 70 mg/dL) or a symptomatic episode that fulfilled Whipple's triad (low serum glucose, hypoglycemic symptoms, and symptom resolution after glucose administration) within 48 hours after admission for severe hypoglycemia diagnosed in the ED.
Continuous data was expressed as mean ± standard deviation (SD) and tested with Student t-test. Categorical data was expressed as frequencies (%) and tested with Chi-Square test or Fisher's exact test. Univariate logistic regression analysis was used to determine the possible risk factors associated with recurrent hypoglycemia. To access the adjusted effect of different variables, we selected the variables either having a p value < 0.2 in the initial univariate results, or clinical important factors into multivariate logistic regression analysis. Multivariate logistic regression analysis with stepwise forward selection was used to control possible confounding variables and to determine the risk factors for developing recurrent hypoglycemia during hospitalization. Data was analyzed using SAS 9.0 (SAS Institute Inc., Cary, NC, USA) and a p value < 0.05 was considered statistically significant.
We identified 504 patients presenting to the ED with severe hypoglycemia during the study period. Of these, 244 patients were hospitalized (Fig. 1). The remaining 260 patients' medical records were also examined and none of those patients were readmitted to the ED for hypoglycemia. Eleven patients did not have type 2 DM or incomplete information and were excluded from analysis. Of the remaining 233 study patients (92 males and 141 females; mean age, 74.1 ± 9.8 years), 74 (31.8%) were categorized as recurrent hypoglycemia and 159 (68.2%) were categorized as non-recurrent hypoglycemia. All patients studied were diagnosed as type 2 DM.
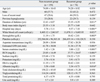
Table 1 presents the comparison of the basic characteristics and laboratory results for the two study groups. There were significant differences of loss of a recent meal, duration of diabetes, duration of hospital stay, initial serum glucose level, WBC, hemoglobin, estimated GFR, serum creatinine, BUN, and albumin among the two groups (p < 0.05). The recurrent hypoglycemia group had a higher percentage of patients (59.5%) with loss of a recent meal (p < 0.001). Patients in the recurrent hypoglycemia group had a significantly longer duration of diabetes and hospital stays than the non-recurrent group. WBC, serum creatinine, and BUN were significantly higher in the recurrent hypoglycemia group as compared to the non-recurrent group. Initial serum glucose level, estimated GFR, and albumin were significantly lower in the recurrent hypoglycemia group as compared to the non-recurrent group.
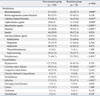
Table 2 presents the medications and comorbidities data. The recurrent hypoglycemia group had significantly higher percentages of patients taking benzodiazepines, alpha-blocker agents, beta-blocker agents, diuretics, and polypharmacy as compared to the non-recurrent group; however, the recurrent hypoglycemia group had a significantly lower percentage of CCBs compared to the non-recurrent group (21.6% vs. 36.1%, p = 0.033). When examining comorbidities, the recurrent hypoglycemia group was found to have a significantly higher prevalence of CAD (60.8%) and infection (47.3%) as compared to the non-recurrent group. There was no statistical significance regarding the different types of oral anti-diabetic medications between the two groups.
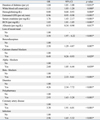
Univariate logistic regression analysis for possible risk factors associated with recurrent hypoglycemia for the 233 subjects are presented in Table 3. Significant risk factors for the development of recurrent hypoglycemia were loss of a recent meal, duration of diabetes, initial serum glucose level, WBC count, hemoglobin, estimated GFR, serum creatinine, BUN, albumin, the use of benzodiazepines, alpha-blocker agents, beta-blocker agents, and diuretics, polypharmacy, and the presence of CAD and infection. The use of CCBs showed a protective effect.
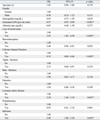
After controlling for age and gender, as shown in Table 4, multivariate logistic regression analysis found that patients with loss of a recent meal (OR = 3.93; 95% CI, 1.82-8.50; p < 0.001), who had CAD (OR = 2.30; 95% CI, 1.04-5.10; p = 0.041) or infection (OR = 2.97; 95% CI, 1.08-6.30; p = 0.032), or who had a renal insufficiency (lower estimated GFR) (OR = 0.97; 95% CI, 0.95-0.98; p < 0.001) were at risk for recurrent hypoglycemia. The use of CCBs (OR = 0.19; 95% CI, 0.08-0.46; p < 0.001) was a protective factor the development of recurrent hypoglycemia.
The purpose of this study is to identify risk factors for recurrent hypoglycemia in diabetic patients hospitalized for severe hypoglycemia. Our study suggests that there is a subset of patients, presenting to the ED with severe hypoglycemia and certain risk factors, who should be admitted due to a greater risk of recurrent hypoglycemia.
About two-thirds of patients with diabetes who were admitted to the hospital due to a hypoglycemic coma did not develope recurrent hypoglycemia. Previous studies have focused on patients treated in the ED who then return for recurrent hypoglycemia. In these studies, the accumulated recurrent hypoglycemia rates has been described as 6.1% by Socransky, et al.,6 9% by Mechem, et al.,14 and 10.14% by Carter, et al.15 We focused on the hospital course of patients admitted due to severe hypoglycemia. As a result, we estimate a rate of recurrent hypoglycemia of 14.7% derived from the number of patients presenting to the ED with hypoglycemia and 31.8% admitted that subsequently developed recurrent hypoglycemia, suggesting a higher rate of recurrent hypoglycemia than previously appreciated.
Once further analyzed with a multiple logic regression analysis, we found that loss of a recent meal was a significant risk factor for recurrent hypoglycemia. Restricted carbohydrate intake had previously been noted as a predisposing factor for drug-induced hypoglycemia.2 Reduced carbohydrate intake can lead to a low energy reservoir, potentiating the development of severe hypoglycemia.2 Renal insufficiency may lead to reduced renal gluconeogenesis and energy intake, as well as a longer half-life of medications that primarily require kidney elimination.16,17 Moreover, CAD and infection were additional risk factors identified. CAD may be a marker for dyslipidemia, which is associated with insulin resistance and metabolic abnormalities. 18 Infection has long been recognized as a risk factor for hyper- and hypoglycemia in diabetic patients.2,9,19 Inflammatory stress from endotoxins,20 fever, and increased metabolism all increase energy consumption. In a prolonged infection, basal glucose production may increase and insulin resistance may develop with or without the presence of hyperglycemia.21,22
The only identifiable protective factor for the recurrence of hypoglycemia was the use of CCBs. It is well known that CCBs are less likely to have metabolic effects. Tsukuda, et al.23 found nifedipine could improve diabetes-associated cognitive impairment. CCBs may also be used in the treatment of hyperinsulinemia hypoglycemia in children.24 However, CCBs as a protective factor for recurrent hypoglycemia has not been reported before, and currently we are unable to explain this unique finding. Further studies are needed to validate this observation.
We have also found that patients who had a longer duration of diabetes, leukocytosis, hypoalbuminemia, the use of certain medications (benzodiazepines, alpha-blockers, beta-blockers, and diuretics), as well polypharmacy, tended to have recurring hypoglycemia during hospitalization. A longer duration of diabetes may be associated with a defect in, or blunting of, the counter-regulatory response to hypoglycemia.9 In addition to poor nutrition, albumin levels are impacted by other factors including inflammation, infection, and trauma. Providing adequate nutrition in the setting of inflammation, infection, or trauma does not improve albumin levels alone; however, inadequate nutrition negatively impacts albumin synthesis. Mediators of inflammatory responses alter hepatic protein synthesis and albumin metabolism leading to a net protein loss followed by anorexia and decreased nutritional intake.9,25 Hypoalbuminemia in the recurrent hypoglycemia group may be a marker for undiagnosed inflammation or infection and for increased morbidity and mortality.9,25 Patients with recurrent hypoglycemia were also at greater risk for longer hospital stays, and hypoalbuminemia has also been associated with longer hospital stays.26 Benzodiazepines, such as alprazolam, have been demonstrated to reduce the neuroendocrine response to insulin-induced hypoglycemia in humans27 and reduce the plasma catecholamine response to exercise stress.28 The long-term use of benzodiazepines may blunt the response to repeated low levels of serum glucose. Several anti-hypertensive agents, such as RASBs, beta-blockers, and diuretics are associated with recurrent hypoglycemia.29-31 Although beta-blockers showed no significant effect after multiple logistic regression in our study, non-selective beta-blockers have been reported to cause severe hypoglycemia in diabetic and non-diabetic patients.32 Beta-blockers may decrease the awareness of hypoglycemia and blunt counter-regulation.30 Using selective beta-blockers may lessen the risk of inducing hypoglycemia.33,34
RASB use in our study population was high (56.7%), and diuretics have been associated with glucose intolerance and insulin resistance in diabetic patients.35,36 Although there are some ADAs with a duration of action as long as 72 hours, we did not find use of these drugs in our study population. We found that polypharmacy was a significant risk factor for recurrent hypoglycemia, consistent with other studies. This is a particular problem for the elderly. Ben-Ami, et al.2, in their study of 102 diabetic patients on ADAs, found that 15% of the 93 patients at risk for a drug-induced hypoglycemic coma were medicated with additional drugs known to potentiate hypoglycemia such as beta-blockers, cimetidine, and aspirin. Shorr, et al.9 in their retrospective cohort study of 586 persons with a first episode of serious hypoglycemia found that the use of 5 or more medications was a risk factor in the development of serious hypoglycemia. No patient was treated with glucagon in this study, since we do not routinely administer glucagon to treat hypoglycemia at our institution, and the emergency medicine technicians are not allowed to use glucagon in Taiwan.
The study design is a non-randomized observational retrospective study with a small study population, and out-of-hospital and ED treatment were also not standardized. There is selection bias, as the patients admitted for severe hypoglycemia were felt to be at greater risk for developing recurrent hypoglycemia as compared to those discharged; however, the reasons for admission were unable to be examined, as there were no standard admission guidelines. As a result, the incidence of recurrent hypoglycemia for all patients presenting to the ED can only be estimated. The underlying reason for the hypoglycemia was not included as a variable, and this may have some impact on the outcomes. Loss of a recent meal could not be calculated accurately because this information was based on the patient's memory (recall bias).
In conclusion, there may be a subset of patients with severe hypoglycemia and certain risk factors for recurrent hypoglycemia who should be admitted. These risk factors include loss of a recent meal, renal insufficiency, CAD, and infection. The use of CCBs seemed to be a protective factor for the development of recurrent hypoglycemia. Further prospective studies regarding the use of these risk factors for the disposition of diabetic patients presenting to the ED with severe hypoglycemia are needed to validate these findings.
Figures and Tables
References
2. Ben-Ami H, Nagachandran P, Mendelson A, Edoute Y. Drug-induced hypoglycemic coma in 102 diabetic patients. Arch Intern Med. 1999. 159:281–284.

3. Kearney T, Dang C. Diabetic and endocrine emergencies. Postgrad Med J. 2007. 83:79–86.
4. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.
5. Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001. 161:1653–1659.
6. Socransky SJ, Pirrallo RG, Rubin JM. Out-of-hospital treatment of hypoglycemia: refusal of transport and patient outcome. Acad Emerg Med. 1998. 5:1080–1085.

7. Lobmann R, Smid HG, Pottag G, Wagner K, Heinze HJ, Lehnert H. Impairment and recovery of elementary cognitive function induced by hypoglycemia in type-1 diabetic patients and healthy controls. J Clin Endocrinol Metab. 2000. 85:2758–2766.

8. Cryer P. Hypoglycemia: Pathophysiology, Diagnosis, and Treatment. 1997. New York: Oxford University Press.
9. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997. 157:1681–1686.

10. The Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes. 1997. 46:1829–1839.
11. Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002. 55:65–85.

12. Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005. 28:1245–1249.
13. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006. 354:2473–2483.

14. Mechem CC, Kreshak AA, Barger J, Shofer FS. The short-term outcome of hypoglycemic diabetic patients who refuse ambulance transport after out-of-hospital therapy. Acad Emerg Med. 1998. 5:768–772.

15. Carter AJ, Keane PS, Dreyer JF. Transport refusal by hypoglycemic patients after on-scene intravenous dextrose. Acad Emerg Med. 2002. 9:855–857.

16. Block MB, Rubenstein AH. Spontaneous hypoglycemia in diabetic patients with renal insuff-iciency. JAMA. 1970. 213:1863–1866.

17. Rutsky EA, McDaniel HG, Tharpe DL, Alred G, Pek S. Spontaneous hypoglycemia in chronic renal failure. Arch Intern Med. 1978. 138:1364–1368.

19. McGuinness OP. Defective glucose homeostasis during infection. Annu Rev Nutr. 2005. 25:9–35.
20. Fong YM, Marano MA, Moldawer LL, Wei H, Calvano SE, Kenney JS, et al. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990. 85:1896–1904.
21. Bell DS. Inflammation, insulin resistance, infection, diabetes, and atherosclerosis. Endocr Pract. 2000. 6:272–276.
22. Saeed M, Carlson GL, Little RA, Irving MH. Selective impairment of glucose storage in human sepsis. Br J Surg. 1999. 86:813–821.
23. Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, et al. Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension. 2008. 51:528–533.

24. Müller D, Zimmering M, Roehr CC. Should nifedipine be used to counter low blood sugar levels in children with persistent hyperinsulinaemic hypoglycaemia? Arch Dis Child. 2004. 89:83–85.
25. Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004. 104:1258–1264.

26. Weinsier RL, Hunker EM, Krumdieck CL, Butterworth CE Jr. Hospital malnutrition. A prospective evaluation of general medical patients during the course of hospitalization. Am J Clin Nutr. 1979. 32:418–426.

27. Giordano R, Grottoli S, Brossa P, Pellegrino M, Destefanis S, Lanfranco F, et al. Alprazolam (a benzodiazepine activating GABA receptor) reduces the neuroendocrine responses to insulin-induced hypoglycaemia in humans. Clin Endocrinol (Oxf). 2003. 59:314–320.

28. Stratton JR, Halter JB. Effect of a benzodiazepine (alprazolam) on plasma epinephrine and norepinephrine levels during exercise stress. Am J Cardiol. 1985. 56:136–139.

29. Morris AD, Boyle DI, McMahon AD, Pearce H, Erans JM, Newton RW, et al. ACE inhibitor use is associated with hospitalization for severe hypoglycemia in patients with diabetes. DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside, Scotland. Medicines Monitoring Unit. Diabetes Care. 1997. 20:1363–1367.

30. Weidmann P, Boehlen LM, de Courten M, Ferrari P. Antihypertensive therapy in diabetic patients. J Hum Hypertens. 1992. 6:Suppl 2. S23–S36.
31. Chelliah A, Burge MR. Hypoglycaemia in elderly patients with diabetes mellitus: causes and strategies for prevention. Drugs Aging. 2004. 21:511–530.
32. Kotler MN, Berman L, Rubenstein AH. Hypoglycaemia precipitated by propranolol. Lancet. 1966. 2:1389–1390.
33. Barnett AH, Leslie D, Watkins PJ. Can insulin-treated diabetics be given beta-adrenergic blocking drugs? Br Med J. 1980. 280:976–978.

34. Pandit MK, Burke J, Gustafson AB, Minocha A, Peivis AN. Drug-induced disorders of glucose tolerance. Ann Intern Med. 1993. 118:529–539.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download