Abstract
Purpose
This study sought to determine whether abdominal obesity is a risk factor for impaired fasting glucose (IFG) and hypertriglyceridemia and to verify whether moderate effect of abdominal obesity on the relationship between IFG and hypertriglyceridemia in Korea.
Materials and Methods
Data from the Korean National Health and Nutrition Examination Survey was used for the analysis. The study population included 5,938 subjects aged 20 year old drawn from non-diabetic participants in a health examination survey. The subjects were classified according to the presence of abdominal obesity based on waist circumference, IFG based on their fasting blood glucose level, and hypertriglyceridemia on their fasting triglyceride.
Results
The multivariate-adjusted odds ratios for the occurrence of hypertriglyceridemia were 2.91 in the abdominal obesity group as compared with the nonobesity group and 1.31 in subjects with IFG compared with the normoglycemia controls. Abdominal obesity was found to be positively moderated in the interaction between waist circumference and fasting blood sugar.
Obesity, hypertension, Type 2 diabetes mellitus (T2DM), and hypertriglyceridemia are risk factors for cardiovascular disease (CVD), and associations among risk factors for CVD have been reported in many studies.1-3 Obesity is a predictor of impaired fasting glucose (IFG), pre-diabetic state, and hypertriglyceridemia.4 Insulin resistance is increased by abdominal obesity, and fasting hyperinsulinemia has been identified as a risk factor for the development of IFG.5-7 It has also been reported that the risk of IFG and T2DM increases with the severity of abdominal obesity. Waist circumference (WC) was the measure of obesity more strongly associated with the metabolic glucose disorders.8 Prevention of IFG and hypertriglyceridemia are closely linked to reduction in the body weight.
The prevalence of T2DM is growing rapidly, the prevalence of the IFG and T2DM were 4.1%, 6.4% in Korea.9 Framingham heart study reported that impaired glucose tolerance (IGT) occurs frequently in obese people and that obese people with IGT are at a high risk of developing T2DM.6
Hypertriglyceridemia is known to be an independent risk factor for cerebrovascular accident (CVA).10 The triglyceride (TG) tends to be related to obesity in comparison with total cholesterol (TC).11 Hypertriglyceridemia is caused by obesity, smoking, drinking, T2DM, and chronic renal failure.12 A 10% increase in weight above ideal body weight was associated with increases in serum cholesterol and fasting blood sugar (FBS) by factors of 12 mg/dL and 2 mg/dL, respectively.13
Gerald studied the relationship between diabetes and hypertriglyceridemia, and reported that hypertriglyceridemia could be caused by the level of very low-density lipoprotein (VLDL) and a TG secretion increase due to diabetes.14 In addition, hypertriglyceridemia differs depending on the degree of glucose intolerance.15 On the other hand, hypertriglyceridemia is an independent risk factor for the development of IFG and T2DM, which was different from Western studies.1
Abdominal obesity is a risk factor of IFG and hypertriglyceridemia. But the causal relationships between IFG and hypertriglyceridemia are not yet clear. Therefore, the present study was performed to determine the relationships between IFG and hypertriglyceridemia in subjects without T2DM and to examine whether there are moderate effects between changes in abdominal obesity according to IFG and hypertriglyceridemia.
This study was based on the 2001 Korean National Health and Nutrition Examination Survey (KNHANES II). Complete data was obtained for 9,770 of 12,647 individuals who participated in the survey. Subjects who had T2DM, had taken medications for T2DM, or had FBS of ≥ 126 mg/dL were excluded. Consequently, 5,938 adults aged 20 years or older were selected for the study.
The KNHANES II is a national survey conducted jointly by the Korea Institute for Health and Social Affairs and the Korea Health Industry Development Institute, and is commissioned by the Ministry of Health and Welfare in accordance with the regulations in the National Health Promotion Act. Selection methods for a nationwide representative sample of Koreans and other survey methods are detailed elsewhere.16,17 The data used in this study was originally provided by the Korea Institute for Health and Social Affairs and the Korea Health Industry Development Institute, and was analyzed again with their permission.
WC was used as a measurement of abdominal obesity. The diagnostic criteria of abdominal obesity is mostly provided for Europeans and North Americans, and the criteria differs depending on race, sex, and age. With reference to the World Health Organization (WHO) Asia-Pacific Obesity Criteria, the normal WC of Asian men was set at < 90 cm and that of women was set at < 80 cm; WC values above this cutoff were classified as abdominal obesity.18
In the case of FBS criteria, the WHO lowered the criteria to 126 mg/dL in accordance with new diagnostic criteria of the American Diabetes Association (ADA) in 1999. FBS levels of 110-125 mg/dL are classified as IFG. The ADA recommended that the diagnostic criteria of IFG should be lowered from 110 mg/dL to 100 mg/dL in 2003.19 With reference to the above levels, FBS values below 100 mg/dL were subsequently classified as normoglycemia, while FBS values of 100-125 mg/dL and above were classified as IFG in this study.
In the case of TG classification, levels below 150 mg/dL and 150 mg/dL and above were defined as normotriglyceridemic and hypertriglyceridemia, respectively, based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III).20
In the evaluation of education level, high school graduation and above was set as the 13th grade, and the education level was classified as either below 13th grade or 13th grade and above. The income level was classified according to the monthly income on the basis of Korean currency (1,000 Won = US$ 1.08).
WC, FBS, and TG were measured during the KNHANES. WC was measured to the nearest 0.1 cm at the narrowest point between the lowest rib and the uppermost lateral border of the right iliac crest. Blood samples were collected from the antecubital vein to measure serum concentrations of TG and glucose after a fast of 10-12 hours.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA) for Windows. Chi-square analyses were used to compare categorical variables. Multiple logistic regression models were constructed for modeling interaction between FBS, WC, and TG. A value of p < 0.05 (two-sided) was taken to indicate statistical significance.
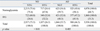
The study population included more females (3,394, 57.2%) than males (2,544, 42.8%). Nonobese subjects comprised 77.4% of the study population, while 22.7% of the subjects had abdominal obesity based on WC. Normoglycemia levels were seen in 68.7% of the participants, and IFG was found in 31.3% on the basis of FBS. Normotriglyceridemic was seen in 67.0% of the participants, and hypertriglyceridemia was seen in 33.0% on the basis of TG (Table 1).
As shown in Table 2, on the basis of WC, the relationship between FBS and TG was examined by classifying the subjects into two groups: nonobesity and abdominal obesity. Compared to subjects with normal FBS levels, nonobese subjects with IFG had a statistically significant higher rate of hypertriglyceridemia (p < 0.001). Additionally, compared to subjects with normal FBS levels, abdominal obesity subjects with IFG had a slightly higher rate of hypertriglyceridemia, but the difference was not statistically significant.
As shown in Table 3, the relationship between WC and TG was examined on the basis of FBS by classifying the subjects into two groups: normoglycemia and IFG. In subjects with IFG, the rate of hypertriglyceridemia was higher in those with abdominal obesity as compared with the nonobese subjects. The result in the normoglycemia group was similar to that of the IFG group. These trends were statistically significant (p < 0.0001).
As shown in Table 4, the relationship between WC and FBS was examined on the basis of TG by classifying the subjects into two groups: normotriglyceridemic and hypertriglyceridemia. In the hypertriglyceridemia group, the incidence of IFG was high in subjects with abdominal obesity as compared with the nonobese subjects. This difference was statistically significant (p < 0.0001). The result in the normotriglyceridemic group was similar to that in the hypertriglyceridemia group.
Multiple logistic regression with hypertriglyceridemia indicated that the dependent variables, WC and FBS, were significantly related. We examined whether there was a moderator effect by interaction between WC and FBS that had an effect on hypertriglyceridemia. The relationship is described in Fig. 1, and the result was statistically significant (p < 0.05) (Table 5).
Various indices can be used to indicate abdominal obesity, and WC has been suggested to reflect visceral obesity better than the waist-to-hip ratio.21 Pouliot, et al.22 suggested that WC is a useful index for the measurement of abdominal fat. Freedman, et al.23 showed that WC has the highest correlation to TG, HDL-C/TC, and systolic blood pressure as compared with other variables for measuring the body, and that it is a risk factor for coronary artery disease (CAD).
WC was also suggested as a criterion of abdominal obesity in NCEP-ATP III. In the case of Western subjects, abdominal obesity is defined as WC of 102 cm and above in men and 88 cm and above in women. According to the recommendations of the WHO Asia-Pacific Region, abdominal obesity in Asians is best defined as a WC of 90 cm or above in men and 80 cm or above in women.11 The cutoff point for the criteria of abdominal obesity differs depending on age, sex, and race. In Korea, abdominal obesity is defined as a WC of 90 cm or above in men and 85 cm or above in women.24 We used the criteria of WHO Asia-Pacific Region for comparison with other countries, the prevalence of abdominal obesity is 22.7% on WC.
WC was a risk factor more strongly associated with the increased levels of IFG and IGT.25,26 Guerrero-Romero, et al.8 reported that the probability of IFG, IGT, and IFG + IGT in obesity were 3.1, 3.2, and 2.8 times higher than in non-obesity, respectively. In this study, abdominal obesity was significantly related to IFG, regardless of hypertriglyceridemia. The odds ratio of having IFG was 2.01 (95% CI: 1.703-2.362, p < 0.05) higher in people with abdominal obesity than in nonobese individuals. The relationship between obesity and diabetes has been examined extensively. IFG is a prediabetic state, and this study clearly demonstrated a relationship between abdominal obesity and glucose metabolism.
The increase of TG in obese patients is due to an increase in VLDL production in the liver and an increase in TG secretion into the blood due to insulin resistance and hyperinsulinemia. The TG rise is caused by increased TG resolution by free fatty acid (FFA), the concentrations of which are high in obese patients.27 Wolf and Grundy28 reported that obese people who lost weight experienced reductions in their TG levels of about 40%.
In the present study, a statistically significant relationship was also seen between abdominal obesity and hypertriglyceridemia regardless of IFG. The odds ratio of hypertriglyceridemia was 2.9 times higher in people with abdominal obesity (95% CI: 2.473-3.426, p < 0.05) than in nonobese subjects. This finding was similar to the results reported previously.27,28
Gerald reported that hypertriglyceridemia is caused by the secretion increase of VLDL and TG due to diabetes.13 The PROCAM study indicated that hypertriglyceridemia and diabetes are independent risk factors for the incidence of CVA.29
Salomaa, et al.15 compared the degree of hypertriglyceridemia according to the degree of glucose intolerance in Finnish subjects aged 45 to 64 years old. In the case of men, hypertriglyceridemia occurred in 47.6% of patients with diabetes, 21.9% with IGT, and 15.4% with normal glucose tolerance (NGT). In the case of women, hypertriglyceridemia occurred in 51.9% of patients with diabetes, 25.7% with IGT, and 10.7% with NGT. These differences were statistically significant after adjusting for age and BMI.
On the other hand, a 9-year longitudinal study performed by Kametani, et al.1 in Japan indicated that hypertriglyceridemia and obesity are independent risk factors for the development of diabetes mellitus. The multivariate-adjusted relative risks for the development of IFG were 1.38 for hypertriglyceridemia and 1.30 for obesity. The relative risk for the development of diabetes mellitus was 1.003 for the TG level.1
St-Pierre, et al.2 reported that IFG did not have an effect on the production of CAD in cases of nonobesity and normotriglyceridemic, but an effect was observed on the incidence of CAD in cases of abdominal obesity and hypertriglyceridemia when IFG was higher.
After controlling for age and abdominal obesity in this study, the odds ratio for hypertriglyceridemia in people with IFG was 1.31 (95% CI: 1.109-1.546, p < 0.05) times higher than in people with a normal glucose level. The results of the present study were also similar to other studies.1,15
Obesity was significantly associated with high serum triglycerides in all glucose tolerance groups.15 Subjects in the present study were classified into nonobesity and abdominal obesity groups. The relationship between IFG and hypertriglyceridemia was examined. IFG and hypertriglyceridemia were significantly related in nonobese individuals, as shown in Table 2, but there was no such relationship in abdominal obesity, indicating that there may be an interaction among WC, FBS, and TG. This was supported by the observation that WC and FBS were significantly associated with the dependent variable of hypertriglyceridemia.
This study has limitations. Because of the cross-sectional nature of the study, our study does not identify a causal relationship. Nevertheless, this study is important to determine the relationships and examine the interactions among abdominal obesity, IFG, and hypertriglyceridemia.
Figures and Tables
 | Fig. 1The interaction effect between FBS and WC on TG. WC, waist circumference; FBS, fasting blood sugar; TG, triglyceride; IFG, impaired fasting glucose. |
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea(A050463).
References
1. Kametani T, Koshida H, Nagaoka T, Miyakoshi H. Hypertriglyceridemia is an independent risk factor for development of impaired fasting glucose and diabetes mellitus: a 9-year longitudinal study in Japanese. Intern Med. 2002. 41:516–521.

2. St-Pierre J, Lemieux I, Vohl MC, Perron P, Tremblay G, Després JP, et al. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease. Am J Cardiol. 2002. 90:15–18.
3. Schmidt MI, Watson RL, Duncan BB, Metcalf P, Brancati FL, Sharrett AR, et al. Atherosclerosis Risk in Communities Study Investigators. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Metabolism. 1996. 45:699–706.

4. Guthrie JR, Ball M, Dudley EC, Garamszegi CV, Wahlqvist ML, Dennerstein L, et al. Impaired fasting glycaemia in middle-aged women: a prospective study. Int J Obes Relat Metab Disord. 2001. 25:646–651.

5. Cassano PA, Rosner B, Vokonas PS, Weiss ST. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. A prospective cohort study of men in the normative aging study. Am J Epidemiol. 1992. 136:1474–1486.

6. Wilson PW, McGee DL, Kannel WB. Obesity, very low density lipoproteins, and glucose intolerance over fourteen years: The Framingham Study. Am J Epidemiol. 1981. 114:697–704.

7. Ryu SH, Beck SH, Chang YS, Kim DI, Suh BS, Kim WS, et al. Abdominal obesity in relation to the incidence of type 2 diabetes mellitus and impaired fasting glucose among some Korean adults: A Retrospective Cohort Study. J Prev Med Public Health. 2004. 37:359–365.
8. Guerrero-Romero F, Rodríguez-Morán M, Pérez-Fuentes R, Sánchez-Guillén MC, González-Ortiz M, Martínez-Abundis E, et al. Prediabetes and its relationship with obesity in Mexican adults: The Mexican Diabetes Prevention (MexDiab) Study. Metab Syndr Relat Disord. 2008. 6:15–23.

9. The Third Korea National Health and Nutrition Examination Survey (KNHANES III) 2005-Health Examination-. 2006. Seoul: Korean Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention;58–62.
10. van Wijk JP, Halkes CJ, Erkelens DW, Castro Cabezas M. Fasting and daylong triglycerides in obesity with and without type 2 diabetes. Metabolism. 2003. 52:1043–1049.

11. Després JP, Allard C, Tremblay A, Talbot J, Bouchard C. Evidence for a regional component of body fatness in the association with serum lipids in men and women. Metabolism. 1985. 34:967–973.

12. Gopal L, Sunder KS, Rao SK, Soni M, Sharma S, Ramakrishnan S. Hyperlipidemia in a poorly controlled diabetic presenting with lipemic aqueous and lipemia retinalis. Retina. 2004. 24:312–315.

13. Kannel WB, Gordon T. Physiological and medical concomitants of obesity: The Framingham Study. Obesity in America. 1979. Washington, D.C.: NIH Publication;125–163.
14. Gerald PS. X-linked mental retardation and the fragile-X syndrome. Pediatrics. 1981. 68:594–595.
15. Salomaa VV, Tuomilehto J, Jauhiainen M, Korhonen HJ, Stengård J, Uusitupa M, et al. Hypertriglyceridemia in different degrees of glucose intolerance in a Finnish population-based study. Diabetes Care. 1992. 15:657–665.
16. Kim SM, Lee JS, Lee J, Na JK, Han JH, Yoon DK, et al. Prevalence of diabetes and impaired fasting glucose in Korea: Korean National Health and Nutrition Survey 2001. Diabetes Care. 2006. 29:226–231.
17. Choi KM, Park HS, Han JH, Lee JS, Lee J, Ryu OH, et al. Prevalence of prehypertension and hypertension in Korean population: Korean National Health and Nutrition Survey 2001. J Hypertens. 2006. 24:1515–1521.

18. World Health Organization (WHO). International Association of the Study of Obesity (IASO). the International Obesity Task Force (IOTF). The Asia-Pacific perspective: redefining obesity and its treatment. 2000. Melbourne: Health Communications Australia;20.
19. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003. 26:Suppl 1. S5–S20.
20. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adults Treatment Panel III) final report. Circulation. 2002. 106:3143–3421.
21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
22. Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994. 73:460–468.

23. Freedman DS, Srinivasan SR, Harsha DW, Webber LS, Berenson GS. Relation of body fat patterning to lipid and lipoprotein concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 1989. 50:930–939.

24. Lee S, Park HS, Kim SM, Kwon HS, Kim DY, Kim DJ, et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J Obes. 2006. 15:1–9.
25. Gómez-Ambrosi J, Pastor C, Salvador J, Silva C, Rotellar F, Gil MJ, et al. Influence of waist circumference on the metabolic risk associated with impaired fasting glucose: effect of weight loss after gastric bypass. Obes Surg. 2007. 17:585–591.

26. Thompson JL, Herman CJ, Allen P, Helitzer DL, Wilson ND, Whyte AN, et al. Associations between body mass index, cardiorespiratory fitness, metabolic syndrome, and impaired fasting glucose in young, urban native american women. Metab Syndr Relat Disord. 2007. 5:45–54.

27. Olefsky JM. Harrison TR, editor. Obesity. Principles of internal medicine. 1991. 12th ed. New York: McGraw-Hill Inc.;411–414. cited from Kook SR, Park YS, Ko YK, Kim SM, Lee DJ, Kang HC, et al. Relationship of body fat, lipid, blood pressure, glucose in serum to waist-hip ratio between obese and normal body mass index group. J Korean Acad Fam Med 1997;18:317-27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download