Abstract
Purpose
Prader-Willi syndrome (PWS) is a genetic disorder characterized by childhood-onset obesity and endocrine dysfunction that leads to cardiovascular disability. The objective of the study is to assess the relationship between carotid intima-media thickness (IMT) and atherosclerotic risk factors.
Materials and Methods
Twenty-seven PWS children and 24 normal controls were enrolled. Correlations of IMT with atherosclerotic risk factors were assessed.
Results
IMTs in the PWS group did not differ from those in the controls (p = 0.172), although total ghrelin levels were higher in the PWS children (p = 0.003). The multivariate analysis revealed positive correlations between total ghrelin levels (ρ = 0.489, p = 0.046) and IMT in the PWS group and between body mass index-standard deviation score (BMI-SDS) (ρ = 0.697, p = 0.005) and IMT in the controls.
Prader-Willi syndrome (PWS) is the most common form of human syndromic obesity resulting from the non-expression of paternal alleles within the PWS region of chromosome 15q11-13.1,2 During childhood, the syndrome is manifested by diminished fetal activity, muscular hypotonia, mental retardation, short stature, small hands and feet, and eventually obesity.3 Although obesity itself is associated with atherosclerosis, literature shows that atherosclerosis in PWS is more serious and earlier in onset than in the general obese population. Premature coronary artery atherosclerosis and sudden cardiac death was reported in a 3-year-old young PWS male.4 Moreover, the natural history of PWS demonstrates that most adult PWS patients have diabetes, dyslipidemia, and cardiopulmonary dysfunctions, which lead to disability and death within the first three decades of life.5
Ghrelin is a 28-amino acid residue peptide predominantly produced by the stomach. Ghrelin signals directly to the hypothalamic regulatory nuclei that control energy homeostasis.6,7 In general, fasting plasma ghrelin levels are lower in obese subjects than in lean controls8,9 and are elevated in patients with anorexia nervosa.10 Fasting increases the plasma level of ghrelin, which then decreases after feeding.11-13 However, grossly elevated plasma ghrelin levels have been demonstrated in PWS adults14 and children.15-17 Interestingly, plasma ghrelin concentrations are reported to be positively associated with carotid artery atherosclerosis in middle-aged males and appear to be a novel risk factor for atherosclerosis.18
Therefore, we hypothesized that elevated plasma ghrelin levels may be one of the risk factors for atherosclerosis in PWS children, which is assessed by carotid artery intimamedia thickness (IMT). An ultrasound measurement of the carotid wall is a surrogate marker of generalized atherosclerosis that correlates with the coronary artery disease and predicts future cardiovascular events in adults.19,20 Moreover, previous observations suggest that the thickening of arterial IMT occurs in children with hypercholesterolemia as well as in children with type 1 diabetes.21,22 Therefore, IMT measurement can be a useful marker for future atherosclerosis even in children.
We measured common carotid artery IMT in children with PWS and in normal control subjects adjusted for age, sex, and body mass index (BMI), and assessed the correlations of IMT with vascular risk factors including sex, age, BMI-standard deviation score (BMI-SDS), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), C-reactive protein (CRP), homeostasis model assessment for insulin resistance (HOMA-IR), and total ghrelin.
We studied twenty-seven children with PWS and 24 normal control subjects. The groups were adjusted for age, sex, and BMI. The clinical characteristics of the study subjects are shown in Table 1.
The PWS subjects were recruited from the outpatient clinic of the Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea. The inclusion criteria were as follows: age of 7-14 years, normotensive, nonsmoker, and absence of any chronic disease. Subjects with diabetes mellitus (i.e., fasting plasma glucose > 126 mg/dL with a 2 hour oral glucose tolerance test value of > 200 mg/dL) were excluded. All of the PWS patients were receiving growth hormones (Genotropin®; Pfizer, New York, NY, USA) in a dose of 1 mg/m2/day (median duration of the growth hormone (GH) treatment: 2.5 years, interquartile range: 2.1-2.9 years). None of the controls were receiving GH therapy. And none of the subjects were taking any other medication except GH.
Tanner stage evaluations were systematically performed in all the children. All of them were prepubertal, and their clinical characteristics are presented in Table 1. The purpose of the study was explained and a written study protocol was sent to all parents or guardians. For the PWS children, informed consent was obtained from the parents or guardians; for the controls, it was obtained from the study participants as well as the parents or guardians. Monetary compensation was not provided to the subjects. The study design was reviewed and approved by the Samsung Medical Center Institutional Review Board.
The carotid artery was examined with a 12- to 5-MHz broadband linear transducer using an HDI 5000 (ATL, Bothell, WA, USA). Longitudinal images directed through the center of the artery were taken for each vessel. The carotid IMT was defined as the maximal distance between the interface of the lumen and the intima and the interface between the media and the adventitia and was recorded at common carotid arteries and averaged for the left and right sides. All diameters were measured during diastole in order to avoid image blurring due to systolic arterial wall motion and minimize the influence of blood pressure.
The total plasma ghrelin levels were measured in duplicate using a commercial ELISA kit (Phoenix Pharmaceuticals, Belmont, CA, USA) and the samples were diluted ten times and the concentrations were re-calculated thereafter: the inter- and intra-assay coefficients of variance were less than 10%, and according to the manufacturer, the lower and upper detection limits for this assay were 40.6 pg/mL and 2,595 pg/mL, respectively. The HOMA-IR was used for calculating the insulin sensitivity index. The body fat percentage of all children was assessed using Lunar Prodigy dual energy X-ray absorptiometry (DXA) (GE Lunar, Madison, WI, USA).
All values are expressed as the median and interquartile ranges in the tables and as means ± SE in the figures. The t-test was used when the data were normally distributed; in other cases, the Mann-Whitney U test was used to compare the other values between the PWS and control groups. In univariate analysis, the correlations were determined using Spearman's correlation analysis because the samples were not normally distributed. In addition, multivariate analysis (Spearman's partial correlation analysis) was performed to reveal variables that are related to IMT independently. The following variables were included in analysis: sex, age, BMI-SDS, HDL, LDL, triglyceride (TG), CRP, HOMA-IR, and total ghrelin. All statistical analyses were performed using SAS version 9.13 (SAS Corp., Cary, NC, USA). p values < 0.05 were considered statistically significant.
Table 1 describes the clinical characteristics of the study subjects. The PWS and control groups did not differ significantly with respect to age or sex ratio, BMI, BMI-SDS, body fat percentage, CRP, and HOMA-IR. However, the levels of HDL cholesterol in the PWS group were higher than those observed in the control group [1.53 mmol/L (1.35-1.74) vs. 1.28 (1.12-1.48), p = 0.004]; the total cholesterol, LDL, and triglyceride levels did not differ between the two groups.
IMTs in children with PWS did not differ from those in the control group [0.04 cm (0.04-0.05) vs. 0.05 (0.04-0.05), p = 0.172]. However, the total ghrelin levels were higher in the PWS group compared with the control subjects [2.92 ng/mL (2.31-4.63) vs. 1.97 (1.10-2.71), p = 0.003]. There was no significant difference between the IMTs of PWS male and female subjects (p = 0.299). Similarly, IMTs in the control males did not differ from those in the control females (p = 0.595).
In univariate analysis, there were correlations of IMT with age (ρ = 0.446, p = 0.019), HDL (ρ = -0.399, p = 0.038) and TG (ρ = 0.638, p = 0.003) in PWS group and BMI-SDS (ρ = 0.516, p = 0.009) in controls (Table 2). However, because there was a strong positive correlation of IMT with TG (ρ = 0.638, p = 0.003) and a negative tendency of correlation of TG with ghrelin (ρ = - 0.222, p = 0.266), the correlation between IMT and ghrelin may be obscured by the relation with TG. Therefore, a multivariate analysis was needed to evaluate the relationship between ghrelin and IMT.
Remarkably, in multivariate analysis, the results revealed the positive correlation of IMT with total ghrelin levels (ρ = 0.489, p = 0.046) in the PWS group (Fig. 1A) and with BMI-SDS (ρ = 0.697, p = 0.005) in the control group (Fig. 1B) (Table 3). However, IMT did not correlate with the ghrelin levels in the control group (ρ = 0.381, p = 0.178). The other variables were not significantly correlated with IMT both in the PWS group and controls.
PWS is a genetic disease that can be caused by deletion, uniparental disomy of chromosome 15q11, or imprinting defects.1,2,23,24 Because this condition first becomes noticeable through hypotonia and poor feeding during the neonatal or infant stages of development, this disorder can be detected at this stage using methylation-specific PCR diagnostic tests. Although infants with PWS appear physically underdeveloped during their early life, they are likely to become obese before reaching school age unless appropriate nutritional guidance and growth hormone treatment is initiated. Natural course study showed that obesity results in type 2 diabetes and early cardiovascular compromise within the first three decades of life.5 It has been reported that PWS subjects suffer from cardiopulmonary dysfunction, which often lead to disability within the first 3 decades of life and early death.5 Diabetes and dyslipidemia, which resulted from excessive obesity, have been blamed for this morbidity and mortality. However, the reported mortality in PWS is far beyond our expectations; thus, further efforts to elucidate the risk factors for atherosclerosis, besides the known ones, were warranted.
Our results did not show that IMTs in children with PWS differ from those in the controls at the age of 10. We speculate that definite carotid intima-media thickening occurs over this age because atherosclerosis is a long time process. A future study will be needed in an adult PWS population to understand the natural atherosclerotic process in PWS. Although the thickening of the carotid artery is not evident in PWS children at the median age of 10 years, our study demonstrates that in elevated levels of plasma ghrelin in children with PWS, which have been reported by our group and several other groups, correlate with IMT of the carotid artery. These findings suggest that these elevated ghrelin levels may be a potential risk factor for carotid artery intima-media thickening in PWS, which may lead to atherosclerosis and coronary artery disease within the first three decades of life.5
We tried to figure out the reason why the correlation between IMT and ghrelin was not obvious in univariate analysis. In univariate analysis, the relation with TG might obscure the correlation between IMT and ghrelin, because there was a strong positive correlation of IMT with TG (ρ = 0.638, p = 0.003) and a negative tendency of correlation of TG with ghrelin (ρ = - 0.222, p = 0.266). Eventually, a multivariate analysis revealed a positive correlation between ghrelin and IMT (ρ = 0.489, p = 0.046).
However, there were a high level of ghrelin and a positive correlation of IMT with the total ghrelin level in PWS children, which is not observed in age-, sex-, and BMI-adjusted controls. This finding may be interpreted as a potential risk factor for carotid intima-media thickening in PWS children. Therefore, considering the positive correlation of IMT with total ghrelin levels and the high level of ghrelin in PWS children, a vigorous effort to lower the known risk factors of atherosclerosis is advocated for patients of PWS in general, and even for the PWS children who are not extremely obese. Previously we reported a greater tendency for the up-regulation of ghrelin level in lean rather than in obese PWS individuals and inverse correlations were found between plasma ghrelin levels and the following: age, BMI percentage of the ideal weight for age, and BMI percentile in PWS children.16
There are few reports that have studied the relationship between plasma ghrelin levels and the risk of atherosclerosis; there was a report demonstrating a positive association between the mean IMT and the ghrelin concentration in the analysis of middle-aged males before and after adjustments for the traditional risk factors of atherosclerosis. 18 Their report and our current study are somewhat unexpected since ghrelin levels have been reported to be negatively associated with BMI. Moreover, obesity itself has been a well-known risk factor for atherosclerosis.25 Therefore, high levels of ghrelin should have been negatively associated with atherosclerosis and accordingly observed as decreased thickness of carotid IMT in this study. However, our study revealed that an elevated level of ghrelin is positively related with carotid IMT independently.
So far, the effect of ghrelin on the vascular system is somewhat controversial. Ghrelin has been reported to improve endothelial dysfunction and increase endothelial nitric oxide synthetase expression in GH-deficient rats and associate with the attenuation of sympathetic nerve activity.26 However, the vasoconstrictive effects of ghrelin, which adversely affect the vascular system, have also been reported.27
Our study is also limited because PWS subjects of this study were treated with GH for 2.5 median years. But Choe, et al.17 have mentioned that hyperghrelinemia cannot be explained by the IGF-1 or GH deficiency and that hyperghrelinemia is not related to GH deficiency state in PWS patients, because hyperghrelinemia is present even after GH replacement.
In summary, at the median age of 10, no difference was observed between the PWS and control groups with respect to IMT; moreover, none of the children displayed plaques or eccentric thickening of the carotid artery. However, there was a high level of ghrelin and a positive correlation of IMT with total ghrelin level in PWS children, which is not observed in age-, sex-, and BMI-adjusted controls. And considering the positive correlation of IMT with total ghrelin levels and the high level of ghrelin in PWS children, a further study will be needed to elucidate the effect of elevated ghrelin levels on the atherosclerosis in patients of PWS group.
Figures and Tables
Fig. 1
Correlation of intima media thickness (IMT) with total ghrelin in the PWS group (A) and BMI-SDS in the controls (B). IMT was significantly positively correlated with total ghrelin in the PWS group (ρ = 0.489, p = 0.046). and BMI-SDS in the controls (ρ = 0.697, p = 0.005). PWS, Prader-Willi syndrome; BMI-SDS, body mass index-standard deviation score.

Table 3
Multivariate Analysis between Adjusted Covariate and Carotid Intima-Media Thickness
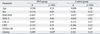
BMI-SDS, body mass index-standard deviation score; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; HOMA-IR, homeostasis model assessment for insulin resistance.
ρ = Spearman partial correlation coefficient, adjusted by each factors.
*p < 0.05.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Korean Health 21 R&D project, Ministry of Health & Welfare (01-PJ10-PG6-01GN15-0001), Samsung Biomedical Research Institute grant (C-A5-215-3) and Samsung Medical Center, Clinical Research Development Program grant (CRS107-12-1).
References
1. Ishikawa T, Kibe T, Wada Y. Deletion of small nuclear ribonucleoprotein polypeptide N (SNRPN) in Prader-Willi syndrome detected by fluorescence in situ hybridization: two sibs with the typical phenotype without a cytogenetic deletion in chromosome 15q. Am J Med Genet. 1996. 62:350–352.
2. MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997. 6:1873–1878.
3. Robinson WP, Bottani A, Xie YG, Balakrishman J, Binkert F, Mächler M, et al. Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am J Hum Genet. 1991. 49:1219–1234.
4. Pomara C, D'Errico S, Riezzo I, de Cillis GP, Fineschi V. Sudden cardiac death in a child affected by Prader-Willi syndrome. Int J Legal Med. 2005. 119:153–157.

5. Lamb AS, Johnson WM. Premature coronary artery atherosclerosis in a patient with Prader-Willi syndrome. Am J Med Genet. 1987. 28:873–880.

6. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000. 141:4325–4328.

8. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001. 50:707–709.

9. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002. 87:240–244.

10. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005. 289:E373–E381.

11. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001. 86:4753–4758.

12. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001. 50:1714–1719.

13. Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001. 24:RC19–RC21.
14. DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in prader-willi syndrome. J Clin Endocrinol Metab. 2002. 87:5461–5464.

15. Haqq AM, Farooqi IS, O'Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003. 88:174–178.

16. Paik KH, Jin DK, Song SY, Lee JE, Ko SH, Song SM, et al. Correlation between fasting plasma ghrelin levels and age, body mass index (BMI), BMI percentiles, and 24-hour plasma ghrelin profiles in Prader-Willi syndrome. J Clin Endocrinol Metab. 2004. 89:3885–3889.
17. Choe YH, Song SY, Paik KH, Oh YJ, Chu SH, Yeo SH, et al. Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader-Willi syndrome. J Clin Endocrinol Metab. 2005. 90:5441–5445.
18. Pöykkö SM, Kellokoski E, Ukkola O, Kauma H, Päivänsalo M, Kesäniemi YA, et al. Plasma ghrelin concentrations are positively associated with carotid artery atherosclerosis in males. J Intern Med. 2006. 260:43–52.
19. Geroulakos G, O'Gorman D, Nicolaides A, Sheridan D, Elkeles R, Shaper AG. Carotid intima-media thickness: correlation with the British Regional Heart Study risk score. J Intern Med. 1994. 235:431–433.
20. Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998. 128:262–269.

21. Yamasaki Y, Kawamori R, Matsushima H, Nishizawa H, Kodama M, Kajimoto Y, et al. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994. 43:634–639.

22. Lavrencic A, Kosmina B, Keber I, Videcnik V, Keber D. Carotid intima-media thickness in young patients with familial hypercholesterolaemia. Heart. 1996. 76:321–325.

23. Mascari MJ, Gottlieb W, Rogan PK, Butler MG, Waller DA, Armour JA, et al. The frequency of uniparental disomy in Prader-Willi syndrome. Implications for molecular diagnosis. N Engl J Med. 1992. 326:1599–1607.

24. Glenn CC, Driscoll DJ, Yang TP, Nicholls RD. Genomic imprinting: potential function and mechanisms revealed by the Prader-Willi and Angelman syndromes. Mol Hum Reprod. 1997. 3:321–332.
25. Heyden S, Cassel JC, Bartel A, Tyroler HA, Hames CG, Cornoni JC. Body weight and cigarette smoking as risk factors. Arch Intern Med. 1971. 128:915–919.
26. Shimizu Y, Nagaya N, Teranishi Y, Imazu M, Yamamoto H, Shokawa T, et al. Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochem Biophys Res Commun. 2003. 310:830–835.

27. Pemberton CJ, Tokola H, Bagi Z, Koller A, Pöntinen J, Ola A, et al. Ghrelin induces vasoconstriction in the rat coronary vasculature without altering cardiac peptide secretion. Am J Physiol Heart Circ Physiol. 2004. 287:H1522–H1529.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download