Abstract
Cytomegalovirus (CMV) infection is usually subclinical in immunocompetent individuals, however it can be life threatening in an elderly immunocompetent individual. We report a case of CMV enteritis causing ileal perforation in a physically active elderly man. An 88-year-old healthy man presented with abdominal pain and diarrhea. After initial conservative treatment, emergency laparotomy was performed for ileal perforation. The diagnosis of CMV enteritis was based on histological findings revealing many large cells with CMV inclusion bodies in the surgical specimen. In elderly individuals, even though they are immunocompetent, CMV enteritis may result in major complications such as bowel perforation, and it should be included in the differential diagnosis of diarrhea if it is resistant to conventional treatment.
Cytomegalovirus (CMV) infection can cause organ-specific damage or severe complications such as bowel perforation in immunocompromised patients.1 On the other hand, CMV infection in an immunocompetent individual is very rare, and only anecdotal reports have described CMV-associated bowel perforation in these patients.2,3 In the present report, we describe a healthy human immunodeficiency virus (HIV)-negative elderly man with CMV enteritis resulting in ileal perforation, and have reviewed the relevant English literature with specific reference to different ages at diagnosis.
An 88-year-old healthy man was admitted with six days history of diarrhea with diffuse abdominal pain. He had no noteworthy past history or any conditions of immunosuppression, including diabetes. He denied any weight loss or fever at admission. His physical examination revealed mild distension and tenderness in the right lower abdomen without rebound tenderness. Laboratory investigation showed leukocytosis of 14.3×106/µL (normal range, 4.0-10.0) and elevated C-reactive protein of 26 mg/dL (normal range, 0.0-5.0). Electrolyte disturbances and azotemia were attributed to dehydration and were easily corrected after rehydration. Blood and stool cultures were negative, and Clostridium difficile toxin was not detected. Widal test and HIV serology were also negative. Colonoscopy revealed no specific lesions from the rectum to the cecum, but intubation into the terminal ileum was failed due to technical difficulty caused by the tortuous course of the colon. The patient began empirical antibiotic therapy for the most probable diagnosis of microbial enteritis. During the first 18 days of hospital stay, his clinical conditions were thought to have improved because his abdominal pain disappeared, white cell count was normalized to 4.7×106/µL and C-reactive protein level was lowered to 1.9 mg/dL.
On the nineteenth hospital day, his general conditions were suddenly aggravated. He had a newly developed fever (38℃), and appeared to be suffering from localized abdominal pain. Palpation of the abdomen revealed tenderness and muscular spasm in the right lower abdomen. Computed tomography of the abdomen revealed wall thickening of the distal ileum and extraluminal air in the peritoneal cavity (Fig. 1). Emergency laparotomy was performed for the presumed diagnosis of peritonitis due to ileal perforation. A segment of ileum, about 25 cm in size, was hyperemic and edematous. One site of bowel perforation was found at the proximal 15 cm from the ileocecal valve. A segment of the ileum including the perforation site was resected and an anastomosis was performed.
The surgical specimen grossly demonstrated reddish ileal mucosa with ulcerations and perforation (Fig. 2). Histopathological examination revealed ulcerations with acute and chronic inflammatory reactions, transmural inflammation around the perforation site, and large cells containing cytoplasmic and nuclear inclusions in the ulcer bed, suggestive of certain viral infections (Fig. 3A). Immunohistochemical staining for CMV showed a positive reaction to the large cells (Fig. 3B). Serologic studies of the CMV infection were negative for CMV IgM, but CMV IgG was 203 AU/mL (upper normal value, < 6 AU/mL). The diagnosis was confirmed as an ileal perforation due to CMV enteritis. The patient recovered without any complications, even though antiviral drugs were not administered after surgical resection. During 15 months follow-up, he has been well without disease recurrence.
The whole gastrointestinal tract can be affected by CMV, however, small bowel was rarely the only site of disease in all reported cases of CMV infection, therefore, this case is unusual in that there was CMV-associated ileal perforation without evidence of colonic lesions. In contrast to CMV infection in immunocompromised hosts,1 only a few reports have described clinically evident CMV infection and their prognosis in immunocompetent individuals.
We reviewed reports of CMV enterocolitis in immunocompetent adults, identified using a computerized search of PubMed database (articles in English between 1992 and 2007) and their references. As there is no consensus on how to define the immunocompetent individuals, the immunocompetence in this analysis was defined with the same definition reported by Galiatsatos, et al.4 as the absence of congenital immune deficiency, acquired immune deficiency syndrom (AIDS), transplantation, prior chemotherapy, or immunosuppressive medication (including corticosteroids). Patients with malignancies or chronic renal failure undergoing hemodialysis were also excluded. Under this definition, 27 of the cases identified by the computerized search were reviewed (Table 1).2,3,5-27 They are composed of 13 men and 14 women with a median age of 60 years. The clinical spectrum ranged from a mild self-limiting colitis to severe complications and death. Surgery was performed in 9 (33%) of 27 patients, who suffered from severe complications including profuse bleeding,19,20 toxic megacolon12 or perforation.2,3,16,18,22,24 Among eight patients more than 70 years old, four (50%) patients underwent laparotomy and 3 (38%) patients were died from severe bacterial sepsis18,20 or multiple organ failure.3 In contrast, overall prognosis in 19 patients less than 70 years old was rather good, as only five (26%) of them underwent laparotomy and none of them was died. In this regard, the prognosis of CMV enterocolitis in immunocompetent individuals morer than 70 years old may be worse and depend mainly on their comorbidities.
The diagnosis of CMV infection has been considered probable by histology of biopsies or detection of more than 4-fold increase in anti-CMV antibody titer and/or CMV-specific IgM. In the meta-analysis of CMV colitis in immunocompetent hosts, only 38.6% had supporting serology.4 Klauber, et al.18 also reported that CMV-IgM antibody was detected in 6 of 13 immunocompetent hosts with CMV colitis. In the cases reviewed here, CMV-IgM antibody was not detected in 6 cases including our patient.3,16,19-21 Therefore, serology is not sufficient to make a timely diagnosis of CMV infection, and the absence of CMV-IgM antibody may not exclude CMV infection. There is no consensus on the use of antiviral drugs, such as ganciclovir, in the treatment of immunocompetent individuals with CMV infection, and CMV enterocolitis resolves spontaneously after surgical resection without antiviral drugs in some immunocompetent cases.2,16,21,24 In this regard, no antiviral drugs were administered to our patient. However, the administration standard requires further discussion in immunocompetent individuals, especially elderly patients.
In elderly individuals, even though they are immunocompetent, CMV enteritis may result in major complications such as bowel perforation, and it should be included in the differential diagnosis of diarrhea if it is resistant to conventional treatment. Serology is not sufficient to make a timely diagnosis of CMV infection, and the absence of CMV-IgM antibody may not exclude acute CMV infection as in our case.
Figures and Tables
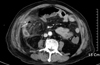 | Fig. 1Abdominal CT scan revealed extraluminal air in the peritoneal cavity (white arrows), reactive fluid collection, and wall thickening of the distal ileum. |
 | Fig. 2Grossly, the specimen revealed one site of bowel perforation (white arrow), measuring about 3×2 cm, at the proximal 15 cm from the ileocecal valve. |
 | Fig. 3Histological examination revealed ulcerations with acute and chronic inflammatory reaction, and large cells (white arrows) containing cytoplasmic and nuclear inclusions at the perforation site (H&E stain, ×400) (A). These large cells showed strong immunoreactivity to CMV antibody (B). CMV, Cytomegalovirus. |
References
1. Frank D, Raicht RF. Intestinal perforation associated with cytomegalovirus infection in patients with acquired immune deficiency syndrome. Am J Gastroenterol. 1984. 79:201–205.
2. Chamberlain RS, Atkins S, Saini N, White JC. Ileal perforation caused by cytomegalovirus infection in a critically ill adult. J Clin Gastroenterol. 2000. 30:432–435.

3. Machens A, Bloechle C, Achilles EG, Bause HW, Izbicki JR. Toxic megacolon caused by cytomegalovirus colitis in a multiply injured patient. J Trauma. 1996. 40:644–646.
4. Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005. 50:609–616.
5. Chau TN, Lau LK, Lee KC, Kwok ML, Lai ST, Yuen H. Association of self-limited cytomegalovirus colitis and shigellosis in an immunocompetent patient. Eur J Gastroenterol Hepatol. 1996. 8:819–822.
6. Lee CS, Low AH, Ender PT, Bodenheimer HC Jr. Cytomegalovirus colitis in an immunocompetent patient with amebiasis: case report and review of the literature. Mt Sinai J Med. 2004. 71:347–350.
7. Lortholary O, Perronne C, Leport J, Leport C, Vildé JL. Primary cytomegalovirus infection associated with the onset of ulcerative colitis. Eur J Clin Microbiol Infect Dis. 1993. 12:570–572.

8. Pfau P, Kochman ML, Furth EE, Lichtenstein GR. Cytomegalovirus colitis complicating ulcerative colitis in the steroidnaive patient. Am J Gastroenterol. 2001. 96:895–899.

9. Maignan M, Wahl D, Thiaucourt D, Bach D, De Korwin JD, Vaillant G, et al. Self-limited primary cytomegaloviris colitis in an immunocompetent individual. J Intern Med. 1992. 232:357–359.

10. Blair SD, Forbes A, Parkins RA. CMV colitis in an immunocompetent adult. J R Soc Med. 1992. 85:238–239.
11. Coll PP, Pacala JT, Hamilton CW. Cytomegalovirus colitis in an older woman, successfully treated with ganciclovir. J Fam Pract. 1992. 34:772–775.
12. Orvar K, Murray J, Carmen G, Conklin J. Cytomegalovirus infection associated with onset of inflammatory bowel disease. Dig Dis Sci. 1993. 38:2307–2310.

13. Consten EC, Brummelkamp WH, Henny CP. Cytomegalovirus infection in the pregnant woman. Eur J Obstet Gynecol Reprod Biol. 1993. 52:139–142.

14. Loftus EV Jr, Alexander GL, Carpenter HA. Cytomegalovirus as an exacerbating factor in ulcerative colitis. J Clin Gastroenterol. 1994. 19:306–309.

15. Tsai HL, Huang CK, Cho G, Chen GH, Yang MD. Cytomegalovirus colitis in an immunocompetent old woman successfully treated with ganciclovir: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1996. 57:289–292.
16. Taniwaki S, Kataoka M, Tanaka H, Mizuno Y, Hirose M. Multiple ulcers of the ileum due to Cytomegalovirus infection in a patient who showed no evidence of an immunocompromised state. J Gastroenterol. 1997. 32:548–552.

17. al Mahdy H. Cytomegalovirus colitis in immunocompetent individual. J Clin Pathol. 1998. 51:475–476.

18. Klauber E, Briski LE, Khatib R. Cytomegalovirus colitis in the immunocompetent host: an overview. Scand J Infect Dis. 1998. 30:559–564.

19. Sakamoto I, Shirai T, Kamide T, Igarashi M, Koike J, Ito A, et al. Cytomegalovirus enterocolitis in an immunocompetent individual. J Clin Gastroenterol. 2002. 34:243–246.

20. Grimsehl K, Seaton RA, Checketts MR. Fulminant cytomegalovirus colitis complicating Staphylococcus aureus septicaemia and multiorgan failure in a previously immunocompetent patient. J R Soc Med. 1999. 92:80–81.

21. Choi SW, Chung JP, Song YK, Park YN, Chu JK, Kim DJ, et al. Lower gastrointestinal bleeding due to cytomegalovirus ileal ulcers in an immunocompetent man. Yonsei Med J. 2001. 42:147–151.

22. Karakozis S, Gongora E, Caceres M, Brun E, Cook JW. Life-threatening cytomegalovirus colitis in the immunocompetent patient: report of a case and review of the literature. Dis Colon Rectum. 2001. 44:1716–1720.
23. Sugisaki K, Maekawa S, Mori K, Ichii O, Kanda K, Tai M, et al. Self-limited colitis during the course of rubella and cytomegalovirus infection in an immunocompetent adult. Intern Med. 2004. 43:404–409.

24. Petrogiannopoulos CL, Kalogeropoulos SG, Dandakis DC, Hartzoulakis GA, Karahalios GN, Flevaris CP, et al. Cytomegalovirus enteritis in an immunocompetent host. Chemotherapy. 2004. 50:276–278.
25. Siegal DS, Hamid N, Cunha BA. Cytomegalovirus colitis mimicking ischemic colitis in an immunocompetent host. Heart Lung. 2005. 34:291–294.
26. Carter D, Olchovsky D, Pokroy R, Ezra D. Cytomegalovirus-associated colitis causing diarrhea in an immunocompetent patient. World J Gastroenterol. 2006. 12:6898–6899.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download