Abstract
Purpose
We investigated the association between the tryptohan hydroxylase 1 (TPH1) gene and aggression in schizophrenia in a Korean population.
Materials and Methods
The sample included 61 aggressive patients as well as 104 non-aggressive patients from psychiatric hospitals and 335 healthy volunteers in Korea. Blood samples were collected from all participants for TPH1 A218C genotyping. The patients were administered standard psychiatric interviews as well as a self-report questionnaire for anger-related traits.
Results
In the case-control phenotypic comparisons, there was no significant association between the aggressive patients and the TPH1 A218C polymorphism. There was no significant effect of the TPH1 genotype on the anger-related traits, or no significant interaction between the genotype and group (aggressive and non-aggressive patients).
Aggressive behavior is frequently reported in patients with schizophrenia, and its prevalence is estimated to be 2 to 10 times that of the general population.1,2 The causes of aggressive behavior in schizophrenia are complex and multifactorial, but a genetic contribution to aggressive behavior has been demonstrated in twin and adoption studies in community sample.3-5 There is a large body of evidence to suggest that disturbances of central serotonin (5-HT) functions play an important role in aggressive behavior.6
The gene responsible for 5-HT synthesis, the tryptophan hydroxylase (TPH) gene, is one of the candidate genes associated with aggression. Several separate lines of biological evidence which suggest its role in modulating serotonergic function in the brain7 together with genetic association studies point to the involvement of TPH1 in aggression or anger-related traits within various psychiatric conditions.8,9 The A218C polymorphism in intron 7 is one of the most widely studied polymorphisms in this gene and could affect the expression of the TPH1 gene by causing a strong linkage disequilibrium with other functional polymorphisms in the promoter region.10 In the previous case-control genetic study by Nolan, et al.,11 a marginal association between TPH1 A218C and aggressive schizophrenia was found, only in male patients, in spite of the small sample size.
Based on the above findings, we investigated whether the genetic variant of TPH1 A218C is associated with aggression in schizophrenic patients. To this end, a case-control phenotypic comparison was carried out, followed by an investigation of anger-related traits as traits affecting aggressive behavior in schizophrenic patients.
The sample included 61 aggressive schizophrenic patients (39 men, 22 women) as well as 104 non-aggressive patients (57 men, 47 women) and 335 healthy volunteers (204 men, 131 women) recruited from the Korean population. The DSM-IV diagnosed schizophrenic patients were recruited to the study between 2004 and 2007 from the inpatients population in Severance hospital, Seoul, and the Chookryoung Evangelical Mental Hospital, Gyeonggi, South Korea. Patients with other comorbid major Axis I psychiatric disorders were not included in this study. The aggressive patients were selected according to rigorous criteria for identifying both a current and past history of aggressive behavior. As described in previous study,12 patients were included in the aggressive group if they had significant episodes of violence, resulting in repeated confinement at least twice per week in the two weeks prior to study inclusion, and a past history of aggressive behaviour defined as a documented history of at least two (and frequently many more) serious assaults against others. Non-aggressive patients were selected for the study if they had no history of significant assaultive or threatening behavior, reported by family members or staff, and if no such behavior was observed during the interview. All of the patients were assessed within 2 weeks after admission and underwent a psychiatric interview conducted by two independent clinicians, based on all available clinical information, examination of case records, and information provided by relatives and mental health professionals. Patients were assessed by the Modified Over Aggression Scale (MOAS)13 for aggressive behavior. We could obtain the data for anger-related traits by the State-Trait Anger Expression Inventory (STAXI)14 in 45 aggressive patients and 84 non-aggressive patients. Of patients, 52 patients in the aggressive group and 82 patients in the non-aggressive group were taking neuroleptics at the time of the study. When the doses of neuroleptics were converted into chlorpromazine equivalent doses,15 there was no significant difference in the dose of neuroleptics between the aggressive and non-aggressive patients. A subgroup consisting of about 30% of the patients within the schizophrenia sample (13 aggressive patients, 32 non-aggressive patients) also completed the assessment of symptoms using the Positive and Negative Syndrome Scale (PANSS) as part of another ongoing study by our group. There was no significant difference between the patients who completed PANSS and those who did not, in terms of age, MOAS or illness variables (onset, duration, hospitalisation and dose of neuroleptics) (data available on request). The exclusion criteria were a history of neurological disorders, substance abuse in the previous 3 months and an estimated intelligence quotient (IQ) of less than 70.
Healthy unrelated Korean volunteers with a mean age of 24.5 years (SD 5.7, range 19-57) were recruited from the residents of Seoul by advertisement. They had no reported current or past history of psychiatric disorders, including significant violent episodes. The Institutional Review Boards of both Severance Hospital and Chookryoung Evangelical Mental Hospital approved the study, and written informed consent was obtained from all participants or their legal guardians after the nature of the study was fully explained to them.
The STAXI14 is a 44-item self-report questionnaire that is divided into seven scales. It measures the current intensity of anger (State anger) and disposition toward anger as a personality trait (Trait anger) using two subscales that measure the subject's general disposition toward angry feelings (Angry temperament) and the tendency to express anger when criticized (Angry reaction). The other scales measure the frequency with which provoked anger is suppressed (Anger-in), the frequency of the expression of anger toward other people or objects (Anger-out), and the frequency at which the expression of anger is controlled (Anger control). The Korean version of the STAXI was used in this study.16 The STAXI has demonstrated satisfactory reliability in schizophrenic patients with Cronbach's α = 0.82-0.85.17
Genotyping was performed blind to history of aggressive behavior. Genomic DNA was extracted from blood leukocytes by using a commercial DNA extraction kit (ABI, Foster City, CA, USA). The genotyping of TPH polymorphism (A218, rs1800532) was carried out using ABI PRISM SNaPShot Multiplex kit (ABI, Foster City, CA, USA) according to manufacturer's recommendation. Briefly, the genomic DNA flanking the SNP for rs1800532 was amplified by polymerase chain reaction (PCR) with the primers 5'-CATGTT CCATGCTCTATATGTGT-3' and 5'-TGTCTGATT TTTTTCAGTGTTACATT-3'. The PCR conditions were as follows: 10 minutes at 95℃ for 1 cycle, and 30 cycles on 95℃ for 30s, 55℃ for 1 min, 72℃ for 1 min followed by 1 cycle of 72℃ for 7 minutes. After amplification, the PCR products were treated with 1 unit each of shrimp alkaline phosphatase (Roche) and exonuclease I (USB Corporation) at 37℃ for 60 minutes and 72℃ for 15 minutes. One µL of the PCR products was added to a SNaPshot Multiplex Ready reaction mixture containing 0.15 pmols of genotyping primer of TPH (5'-TTATTAATTGACAACCTATTAGGTG-3') for primer extension reaction. The primer extension reaction was carried out for 25 cycles of 96℃ for 10 seconds, 50℃ for 5 seconds, and 60℃ for 30 seconds. The reaction products were treated with 1 unit of SAP at 37℃ for 1 hour and 72℃ for 15 minutes to remove excess fluorescent dye terminators. One µL of the final reaction sample containing the extension products was added to 9 µL of Hi-Di formamide (ABI, Foster City, CA, USA). The mixture was incubated at 95℃ for 5 minutes, followed by 5 minutes on ice and then analyzed by electrophoresis in ABI Prism 3730xl DNA analyzer. Results were analyzed using GeneScan analysis software (ABI, Foster City, CA, USA). Deviation from Hardy-Weinberg equilibrium was assessed as described by Wigginton, et al.18
The differences in the genotype and allele frequencies between the affected (aggressive patients) and unaffected individuals (non-aggressive patients or healthy volunteers) were evaluated using a χ2 test. To test the interaction between the genotypes and group (aggressive patients and non-aggressive patients) on anger-related traits, multivariate analysis of variance (MANOVA) was performed, followed by univariate analysis. Gender was entered as a covariate in all analyses to control the possible gender effect on the genotype.19 p-values < 0.05 were considered as significant. All statistical tests were performed using SPSS version 11.0.
The demographic and clinical characteristics of the patients are shown in Table 1. As shown, the aggressive and non-aggressive patients did not differ in age, age at onset or educational level. As expected, the aggressive patients had significantly higher scores in MOAS and STAXI than the non-aggressive patients. In addition, the positive scale [24.7 (9.8) vs. 14.5 (8.2); t = 3.7, df = 43, p = 0.001], negative scale [19.5 (7.0) vs. 13.2 (7.1); t = 2.8, df = 43, p = 0.007], general subscale [48.4 (16.7) vs. 28.3 (12.4); t = 4.5, df = 43, p < 0.001], and total PANSS scores [92.0 (30.8) vs. 56.7 (25.2); t = 4.0, df = 43, p < 0.001] of the aggressive patients were significantly higher than those of the non-aggressive patients.
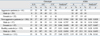
The genotype and allele frequencies of the variants for the aggressive and non-aggressive patients with schizophrenia are shown in Table 2, along with those for the healthy controls. The TPH1 A218C genotype frequencies in both the healthy controls and schizophrenic patients did not deviate from Hardy-Weinberg equilibrium. There were no significant differences in the genotype or allele frequencies between the aggressive patients and controls (non-aggressive patients or healthy volunteers). When the analysis was stratified by gender, the genotype and allele frequencies in the aggressive, non-aggressive patients groups and healthy volunteers were not significantly different.
A MANOVA was computed for patients with schizophrenia by integrating the seven STAXI subscales as well as three factors: the genotype, group and gender. We could not find any effect of genotype on the anger-related traits or any interaction among the genotype, groups, and gender (Table 3). In further detailed univariate analysis followed for genotype effects, there was no effect of genotype on the STAXI subscales in aggressive or non-aggressive patients (Table 4).
In the present study, tests of association with a case-control phenotypic comparison and continuous outcome for anger-related traits revealed no evidence of an association between the TPH1 A218C gene and aggression in schizophrenic patients.
To the best of our knowledge, this is the first study to systematically investigate the association between the tryptophan hydroxylase gene and aggressive schizophrenia. It is interesting to note that our findings are incongruent with those of a previous study in a Caucasian sample.11 One explanation for the discrepancy between our findings and others may be the small sample sizes of aggressive patients in both studies: 61 and 42, respectively, coupled with the fact that the previous positive finding was found in the sample of 34 male patients. Since it is beyond the means of most investigators to gather an adequate sample size of aggressive schizophrenic patients, meta-analyses of well-prepared small studies should be conducted in the future.
In this study, the TPH1 genotype had no effect on anger-related traits in either the aggressive or non-aggressive schizophrenic patients. In our previous study, aggressive behavior was more frequent in schizophrenic patients who were prone to becoming angry.20 Based on our findings, it is suspected that other serotonin-related genes, such as serotonin transporter-related gene,21 or other TPH gene, TPH2, which is considered to control serotonin synthesis in the brain,22 may play a role in aggressive behavior via anger-related traits in schizophrenic patients. On the other hand, as the A218C single nucleotide polymorphism is not considered to be the sole determinant of the functionality of the TPH1 gene,10,23 an analysis of the effect of each haplotype on the expression of the TPH1 gene is also required, in order to clarify the involvement of the TPH gene in aggressive schizophrenia.
The major strength of the current study is its use of rigorous criteria to define the phenotype. Thus, although the recruitment period was twice long as initially planned because aggressive patients who met the criteria were rare, the relatively homogenous phenotype of the patients adds strength to the findings in this study. This study has a few limitations which need to be taken into consideration, however. The first limitation is the relatively small sample size, as already pointed out above. Small sample sizes can lead to both type 1 and type 2 errors. The power of our sample was 53% between aggressive patients and healthy controls, and 42% between aggressive patients and non-aggressive patients, to detect an allele frequency difference of 10% at alpha = 0.05 (two-tailed). The second major limitation is that a self-rating instrument was used to measure anger-related traits. However, we did not think that this led to biased results, because the STAXI has demonstrated high reliability in schizophrenic patients in other studies.15 A third limitation is that the severity of illness could have affected the genotype findings. However, it seems unlikely to be a confounding factor, as there was no association between the PANSS score and TPH genotype in our subsidiary analysis. A fourth is that ages of patients with schizophrenia and control subjects were not matched.
In conclusion, we could not find any association between the genetic variations of TPH1 A218C and aggression in Korean schizophrenia population in the present study. Despite the present findings, there is no doubt that genetic variants remain an important factor in aggressive behavior. Further studies are needed to identify the susceptibility gene in patients with schizophrenia and other clinical or non-clinical samples using well-characterized aggression phenotypes.
Figures and Tables
Table 2
Frequency of Genotypes and Alleles for the TPH1 A218C Gene in Aggressive Schizophrenic Patients, Non-Aggressive Schizophrenic Patients and Healthy Volunteers, Stratified by Gender
ACKNOWLEDGEMENTS
This work was supported by the Biomedical Brain Research Center, Ministry of Health & Welfare, Republic of Korea (01-PJ8-PG6-01NE01-0003) and a 2007 Inje University research grant.
References
1. Hafner H, Boker W. Crimes of violence by mentally abnormal offenders: a psychiatric and epidemiological study in the federal german republic. 1982. Cambridge: Cambridge University Press.
2. Wessely S. The epidemiology of crime, violence and schizophrenia. Br J Psychiatry Suppl. 1997. 8–11.

3. Deater-Deckard K, Plomin R. An adoption study of the etiology of teacher and parent reports of externalizing behavior problems in middle childhood. Child Dev. 1999. 70:144–154.
4. Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: results from two twin studies. Child Dev. 1999. 70:155–168.
5. Hudziak JJ, van Beijsterveldt CE, Bartels M, Rietveld MJ, Rettew DC, Derks EM, et al. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behav Genet. 2003. 33:575–589.
7. Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000. 5:32–38.
8. Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999. 45:603–614.

9. Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Möller HJ. Association of anger-related traits with SNPs in the TPH gene. Mol Psychiatry. 2002. 7:1023–1029.

10. Lai TJ, Wu CY, Tsai HW, Lin YM, Sun HS. Polymorphism screening and haplotype analysis of the tryptophan hydroxylase gene (TPH1) and association with bipolar affective disorder in Taiwan. BMC Med Genet. 2005. 6:14.
11. Nolan KA, Volavka J, Lachman HM, Saito T. An association between a polymorphism of the tryptophan hydroxylase gene and aggression in schizophrenia and schizoaffective disorder. Psychiatr Genet. 2000. 10:109–115.

12. Kim YR, Kim JH, Kim SJ, Lee D, Min SK. Catechol-O-methyltransferase Val158Met polymorphism in relation to aggressive schizophrenia in a Korean population. Eur Neuropsychopharmacol. 2008. 18:820–825.

13. Kay SR, Wolkenfeld F, Murrill LM. Profiles of aggression among psychiatric patients. II. Covariates and predictors. J Nerv Ment Dis. 1988. 176:547–557.
14. Spielberger CD. State-Trait Anger Expression Inventory: professional manual. 1988. Odessa, FL: Psychological Assessment Resources.
15. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol. 2004. 24:192–208.

16. Chon KK. Development of the Korean State-Trait Anger Expression Inventory. Korean J Rehabil Psychol. 1996. 3:53–69.
17. Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol. 2004. 14:267–273.

18. Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005. 76:887–893.

19. Porter RJ, Mulder RT, Joyce PR, Miller AL, Kennedy M. Tryptophan hydroxylase gene (TPHI) and peripheral tryptophan levels in depression. J Affect Disord. 2008. 109:209–212.

20. Song H, Min SK. Aggressive behavior model in schizophrenic patients. Psychiatry Res. 2009. In press.

21. Kim YR, Jahng JW, Min SK. Association between the serotonin transporter gene (5-HTTLPR) and anger-related traits in Korean schizophrenic patients. Neuropsychobiology. 2009. In press.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download