Abstract
Purpose
The pathophysiology of hypogammaglobulinemia in nephrotic syndrome (NS) remains unknown. We evaluated the differences in the distribution of anti-bacterial antibodies and anti-viral antibodies, and those of immune antibodies and natural antibodies in steroid-sensitive NS.
Materials and Methods
We examined the antibody status of 18 children who had routine vaccinations. The levels of immnunoglobulin G (IgG), the IgG subclasses, and the antibodies induced by vaccinations such as diphtheria-pertussis-tetanus and measles-mumpsrubella were analyzed in children with steroid-sensitive NS.
Results
There was a positive correlation between the albumin and IgG values (r = 0.6, p < 0.01), and the four IgG subclasses were all evenly depressed in the nephrotic children during the acute stage of the disease. The antibodies induced by bacterial antigens were depressed and the seropositivity of anti-viral antibodies tended to be lower than those of age-matched control children during the acute stage. The depressed immune antibody status recovered rapidly in the remission stage of NS, despite corticosteroid treatment.
Nephrotic syndrome (NS) is characterised by proteinuria, hypoalbuminemia, hyperlipidemia, and generalized edema. Hypogammaglobulinemia, i.e., low immunoglobulin G (IgG), has also been well documented, but its pathophysiology remains unknown.1,2 Although hypogammaglobulinaemia in NS is regarded as a risk factor for infections, especially with bacterial species such as Streptococcus pneumoniae (S. pneumoniae),3 there have been few studies of the increased risk of other infections, including those of viral in origin, in children with NS.
Since children with minimal-change nephrotic syndrome (MCNS) have highly selective proteinuria,4,5 as do those with congenital NS,6,7 the urinary loss of IgG is negligible when compared to that of other glomerulopathies. Thus, instead of the urinary excretion of IgG, other factors have been suggested to explain the hypogammaglobulinaemia of MCNS, such as the increased catabolism of IgG or reduced IgG synthesis.8-10 It is reported that IgG levels correlate positively with albumin levels in children in the acute stage of MCNS. This finding suggests that IgG levels in NS reflect the severity of the urinary loss of proteins other than IgG.11
IgG is the most abundant of the immunoglobulins and constitutes approximately one third to one half of the proteins in human plasma. Total IgG is composed of four subclasses that differ in their biological functions. It has been proposed that antibodies directed against bacteria or viruses are distributed differently in the IgG subclasses; antibodies directed against viruses are classified in the IgG1 and IgG3 subclasses, and antibodies directed against bacterial agents in the IgG2 subclass.12,13 In addition, IgG may be functionally divided into two classes: immune antibodies that react with both pathogens and foreign antigens, and natural antibodies that react with various self antigens and may exert an immunoregulatory effect on immune-mediated diseases.14-16
In this study, to evaluate the differences in the distributions of immune antibodies and natural antibodies, and differences in the distributions of anti-bacterial antibodies and anti-viral antibodies, we examined the levels of the IgG subclasses and various antibodies induced by vaccinations in children with MCNS.
We evaluated 18 patients with steroid-responsive NS (14 boys and 4 girls, aged between 3 and 14 years) attending the Department of Pediatrics, The Catholic University of Korea, Daejeon St Mary's Hospital. As almost all of the children with steroid-sensitive NS have pathohistologically minimal change lesions, we regard our subjects as having MCNS without conducing a renal biopsy. However, children with steroid-resistant NS or other biopsy-proven glomerulopathies were excluded from the study since these disorders have the possibility of having poorly selective proteinuria. The data was obtained at the initial presentation of each child and included serum levels of albumin, cholesterol, and immunoglobulins (IgG, IgM, IgA, and IgE). All children had serum albumin ≤ 2.5 g/dL and proteinuria > 40 mg/m2/h. As the initial treatment, prednisolone was administered for 4 weeks at a dose of 60 mg/m2 per day or deflazarcort (Calcort®, Sanofi-Aventis Korea, Seoul, Korea) at an equivalent dose, and all children experienced complete remission. An alternate-day therapy was then given at a dose of 40 mg/m2, combined with a tapering of the dose for 3-4 months. The urine protein selectivity index (IgG to transferrin ratio) and 24-hour urine protein electrophoresis were evaluated in eight children. Sera from children with MCNS at presentation and at remission (1-2 months after the initiation of steroid therapy) were stored at -70℃ and used for the evaluation of the IgG subclasses, antibodies induced by vaccinations for diphtheria-pertussistetanus (DPT) and measles-mumps-rubella (MMR), and anti-Epstein-Barr virus viral capsid antigen IgG (anti-EBV VCA IgG). Sera of 20 age-matched control subjects were prepared from healthy children who had minor elective surgery in our hospital. In Korea, it is assumed that most children over 5 years of age have been infected with EBV. All patients and the control children had a routine vaccination history for DPT (4-5 doses) and MMR. The IgG subclasses were measured by the nephelometric method (The Binding Site Ltd, San Diego, CA, USA). The seropositivity of anti-viral and anti-pertussis antibodies was measured by commercial kits, and the anti-diphtheria IgG and antitetanus IgG were analysed by quantitative method. Anti-measles IgG was measured by enzyme-linked immunoassay (EUROIMMUN, Lübeck, Germany), anti-mumps IgG by indirect immunofluorescence assay (BION Enterprise, Des Plaines, IL, USA), anti-rubella IgG by enzymelinked immunoassay (Abbott Laboratories, Abbott Park, IL, USA) and anti-VCA IgG by an indirect immunofluorescence assay (BION Enterprise, Des Plaines, IL, USA). Antibodies directed against the bacterial antigens (anti-diphtheria IgG, anti-tetanus IgG and anti-pertussis IgG) were measured by enzyme-linked immunosorbent assay (Im-muno-Biological Laboratory Inc. Minneapolis, MN, USA). The study was approved by the Ethics Committee for Clinical Research at The Catholic University of Korea, Daejeon St. Mary's Hospital.
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 12.0 (SPSS, Inc, Chicago, IL, USA). The means of continuous variables (the values for the IgG subclasses, anti-diphtheria, and anti-tetanus IgGs) between the acute stage and the remission stage of NS were compared using the paired t-test, and using the Mann-Whitney U test between NS groups and the control group. For categorical data (seropositivity), χ2 was used. A p ≤ 0.05 was considered statistically significant.
The mean age of the 18 nephrotic children was 6.5 ± 3.0 years. The mean values for albumin, IgG, and total cholesterol in the nephrotic children were 1.8 ± 0.4 g/dL, 398 ± 138 mg/dL, and 393 ± 96 mg/dL, respectively. At admission, the levels of IgM, IgA, and IgE were 298 ± 152 mg/dL, 155 ± 55 mg/dL, and IgE 823 ± 798 IU/mL, respectively. These results indicate that the IgM and IgE levels were elevated compared to the normal reference ranges for age.
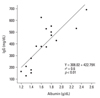
There was also a negative correlation between the serum albumin and total cholesterol values (r = -0.6, p < 0.01), and a positive correlation between the albumin and IgG values (r = 0.6, p < 0.01) (Fig. 1), as previously observed.11 All eight nephrotic children analysed for the urinary protein selectivity index (IgG to transferrin) and 24-hour urine protein electrophoresis had a highly selective index of < 0.1, and the excretion of γ-fraction proteins in their urine was < 3%. The values for IgG and the IgG subclasses at presentation and at remission in the children with NS and those of the control children (mean age 6.9 ± 3.1 years) are shown in Table 1. The values for total IgG and the four IgG subclasses were all significantly reduced during the acute and remission stages compared to those of the control children. However, the levels of IgG3 and IgG4 in the acute stage were not statistically different from those in the remission stage (Table 1).
The rates of seropositivity, the levels of antibodies induced by vaccinations, and the levels of anti-VCA IgG are shown in Tables 2 and 3. The rates of seropositivity for anti-viral antibodies during the acute stage were lower than those of the control children, but there was no statistically significant difference overall antibodies (Table 2). Of the anti-bacterial antibodies, the values for anti-diphtheria IgG, anti-tetanus IgG during the acute stage were significantly lower than the corresponding values during remission, and significantly lower than those of the control children. However, there was no difference between those of the nephrotic children during remission and those of the control children. The rate of seropositivity for anti-pertussis antibodies in the nephrotic children was lower than that of the control children at acute stage (33% vs. 60%), but statistically not significant like the viral antibodies (Table 3).
Although the immunopathogenesis of various renal diseases that can induce NS differs according to the disease, dysgammaglobulinaemia (low IgG, but high IgM and IgE levels) is a common feature in all nephrotic patients, including those with congenital NS which is caused by genetic defects in the glomerular basement membrane.1,2,6,7 In the present study, we found that the level of albumin correlated with the level of IgG, and that there was a negative correlation between albumin and total cholesterol levels during the acute stage of MCNS, as reported in a previous study.11 In addition, these phenomena were observed rather rapidly within several days when relapsed children with NS were evaluated.11 Thus, these findings suggest that the hypogammaglobulinemia in NS may not be associated with the immunopathogenesis of NS; instead, it may represent a secondary phenomenon, such as hyperlipidaemia, arising from the disturbance of protein metabolism in NS. Although many studies have focused on the increased catabolism of IgG, including urinary loss,8,17 the highly selective proteinuria of MCNS and the correlation of IgG and albumin levels during the acute stage of MCNS do not support the urinary loss of IgG.
Since the clinical and laboratory characteristics of NS (hypercholesterolaemia and hypogammaglobulinaemia) result from the urinary loss of medium-sized proteins, mainly albumin, the compensatory mechanisms for this disturbance of protein homeostasis in NS may be induced as soon as the beginning of massive proteinuria. Patients with NS and nephrotic animals may overproduce high-molecular weight proteins, such as lipoproteins, which cause hyperlipidemia and are protected from urinary loss for maintaining the oncotic pressure.18 In addition, in contrast to albumin, an infusion of high-dose IVIG (2 g/kg), even repeated infusions (4 g/kg or more), to patients with Kawasaki disease who maintain normal IgG levels in the acute stage, appears to induce little intravascular volume expansion.19 This finding suggests that IgG does not have an important role in the regulation of the oncotic pressure.15 The rapid correlation of albumin and IgG levels in the acute stage of NS presents the possibility that the reduction of IgG occurs to provide space for other larger proteins involved in the maintenance of intravascular oncotic pressure.
IVIG has been extensively used as a treatment for immune-mediated diseases, including some renal diseases such as lupus nephritis, although nephrotoxicity can be a serious but rare complication.20 The effectiveness of IVIG in a variety of immune-mediated disorders has suggested that IgG in plasma can be divided into two functional classes: immune antibodies and natural antibodies that exert immunoregulatory effects on immune-mediated disorders. Although the methods for distinction between the two antibodies are not yet discovered, it is believed that the majority of IgGs in plasma are the natural antibodies.14-16 However, there is few data on the distribution of these functional IgGs in NS. In the present study, we found that all IgG antibodies (IgG subclasses) were depressed including immune antibodies (both anti-viral and anti-bacterial antibodies) in acute stage of NS.
Hypogammaglobulinaemia in NS has been proposed to be a risk factor for bacterial infections,3 but the data for increased risk of viral infections in nephrotic children are limited. Although antibodies against pathogens are distributed in different IgG classes according to viruses or bacteria,12,13 in this study the levels of all the IgG subclasses showed rather homogeneous depression during the acute stage of MCNS as with previous studies.2,21,22 The seropositivity rates for anti-viral antibodies tended to decrease in this study; however, this finding may indicate that the depressed anti-viral antibodies in the acute stage of NS have not fallen below the threshold for seropositivity. The same pattern of seropositivity in anti-pertussis antibodies with depressed levels of other two bacterial antibodies was also observed (Table 3). Since we did not quantify the antiviral antibodies, further studies with larger samples are required to determine whether there is a difference in the degree of depression of anti-bacterial and anti-viral antibodies. Given the rapid recovery of antibody titers in remission and the important role of cell-mediated immunity for viral infections, the seroconversion of viral antibodies in the acute stage may not indicate a loss of immunity against the invading viruses.
It has been reported that the levels of IgG3 and IgG4 in the acute stage are not statistically different from those in the remission stage,2,21 and we confirmed this finding in this study (Table 1). Although the association between IgG subclasses and some glomerulopathies has been reported,22-24 the dysgammaglobulinemia (including the IgG subclasses, IgM and IgE) and its clinical implications in NS during the acute and remission stages have remained unresolved.
Children with NS show a normal immune response to vaccines, including that for S. pneumoniae, during remission.25,26 Thus, the immunological status of MCNS patients, including their immunological memory for antigens, may be intact although the pathogenesis of MCNS may be related to T-cell dysfunction.27 Humoral immunity (antibodies) plays a crucial role in the phagocytosis of encapsulated bacteria, such as S. pneumoniae. Together with hypogammaglobulinaemia, other factors such as immunosuppressant treatments with corticosteroids or cytotoxic drugs and the urinary loss of immune substances, including those that participate in phagocytosis (e.g., complement), may also be responsible for the high risk of bacterial infections and the aggravated symptoms of some viral infections in patients with MCNS.
In conclusion, we found that IgG levels correlated positively with albumin levels and the levels of all antibodies, immune antibodies and natural antibodies, were reduced during the acute stage of MCNS and recovered after the cessation of proteinuria. Our results indirectly suggest that hypogammaglobulinaemia in NS may be associated with systemic protein homeostasis in vivo. Further studies are required to explain the hypogammaglobulinaemia of patients with NS.
Figures and Tables
Table 1
Serum IgG and IgG Subclass Levels in Children with Minimal Change Nephrotic Syndrome (MCNS) and Age-Matched Controls

References
1. Giangiacomo J, Cleary TG, Cole BR, Hoffsten P, Robson AM. Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal-change nephrotic syndrome. N Engl J Med. 1975. 293:8–12.
2. Kemper MJ, Altrogge H, Ganschow R, Müller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002. 17:413–417.

3. Krensky AM, Ingelfinger JR, Grupe WE. Peritonitis in childhood nephrotic syndrome: 1970-1980. Am J Dis Child. 1982. 136:732–736.
4. Brocklebank T, Cooper EH, Richmond K. Sodium dodecyl sulphate polyacrylamide gel electrophoresis patterns of proteinuria in various renal diseases of childhood. Pediatr Nephrol. 1991. 5:371–375.

5. Ramjee G, Coovadia HM, Adhikari M. Sodium dodecyl sulphate polyacrylamide gel electrophoresis of urinary proteins in steroidresponsive and steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. 1994. 8:653–656.

6. Harris HW Jr, Umetsu D, Geha R, Harmon WE. Altered immunoglobulin status in congenital nephrotic syndrome. Clin Nephrol. 1986. 25:308–313.
7. Huttunen NP, Savilahti E, Rapola J. Selectivity of proteinuria in congenital nephrotic syndrome of the Finnish type. Kidney Int. 1975. 8:255–261.

8. Beaman M, Oldfield S, MacLennan IC, Michael J, Adu D. Hypogammaglobulinaemia in nephrotic rats is attributable to hypercatabolism of IgG. Clin Exp Immunol. 1988. 74:425–430.
9. Heslan JM, Lautie L, Intrator C, Blanc C, Lagrue G, Sobel AT. Impaired IgG synthesis in patients with the nephrotic syndrome. Clin Nephrol. 1982. 18:144–147.
10. Warshaw BL, Check IJ, Hymes LC, DiRusso SC. Decreased serum transferrin concentration in children with the nephrotic syndrome: effect on lymphocyte proliferation and correlation with serum immunoglobulin levels. Clin Immunol Immunopathol. 1984. 33:210–219.
11. Kwak GY, Lee KY, Kim DU, Koh DK, Lee JS. Correlation between serum albumin and IgG level in minimal change nephrotic syndrome. J Korean Soc Pediatr Nephrol. 2007. 11:16–23.
12. Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980. 303:178–182.

13. Linde GA. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985. 21:117–121.

14. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001. 345:747–755.

15. Lee KY, Lee JS. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med Hypotheses. 2006. 67:848–855.

16. Sibéril S, Elluru S, Negi VS, Ephrem A, Misra N, Delignat S, et al. Intravenous immunoglobulin in autoimmune and inflammatory diseases: more than mere transfer of antibodies. Transfus Apher Sci. 2007. 37:103–107.
17. al-Bander HA, Martin VI, Kaysen GA. Plasma IgG pool is not defended from urinary loss in nephrotic syndrome. Am J Physiol. 1992. 262:F333–F337.

19. Lee KY, Han JW, Lee JS. Kawasaki diasease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007. 69:642–651.

20. Orbach H, Tishler M, Shoenfeld Y. Intravenous immunoglobulin and the kidney--a two-edged sword. Semin Arthritis Rheum. 2004. 34:593–601.

21. Warshaw BL, Check IJ. IgG subclasses in children with nephrotic syndrome. Am J Clin Pathol. 1989. 92:68–72.

22. Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome. Eur J Immunol. 1995. 25:1497–1501.
23. Rostoker G, Pech MA, Del Prato S, Petit-Phar M, Ben Maadi A, Dubert JM, et al. Serum IgG subclasses and IgM imbalances in adult IgA mesangial glomerulonephritis and idiopathic Henoch-Schoenlein purpura. Clin Exp Immunol. 1989. 75:30–34.
24. Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997. 51:270–276.

25. Wilkes JC, Nelson JD, Worthen HG, Morris M, Hogg RJ. Response to pneumococcal vaccination in children with nephrotic syndrome. Am J Kidney Dis. 1982. 2:43–46.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download