Abstract
Mycobacterium abscessus (M. abscessus) is the second most common nontuberculous mycobacteria (NTM) in South Korea. Nevertheless, the diagnosis and treatment of M. abscessus lung disease can be problematic. Surgical resection has been tried for patients with localized M. abscessus lung disease refractory to medical treatment. Here, we report on a 25-year-old woman with M. abscessus lung disease who had been diagnosed and treated three times for pulmonary tuberculosis. She was initially diagnosed as having M. intracellulare lung disease; however, M. abscessus was isolated after several months of medication. She had multiple bronchiectatic and cavitary lesions bilaterally, and M. abscessus was repeatedly isolated from her sputa despite prolonged treatment with clarithromycin, ethambutol, moxifloxacin, and amikacin. She improved only after sequential bilateral lung resection. Based on the experience with this patient, we suggest that, if medical treatment fails, surgical resection of a diseased lung should be considered even in patients with bilateral lesions.
Diseases attributable to nontuberculous mycobacteria (NTM) are on the rise, and NTM is responsible for an increasing proportion of mycobacterial diseases in many developed countries.1,2 In South Korea, the number of NTM isolates increased from 448 in 1992 to 1,737 in 2002, while the total number of mycobacterial colonies referred to the Korean Institute of Tuberculosis has decreased from 18,970 to 5,181 in the same period.3 NTM can cause lung disease in an immunocompetent host as well as in an immunocompromised host such as an acquired immune deficiency syndrome (AIDS) patient.2 Decisions regarding the diagnosis and treatment of NTM pulmonary disease can be problematic because clinical findings, radiographic characteristics, and microbiological culture results must be carefully considered to exclude other possible etiologies.1-4
Most species of NTM are resistant to first-line anti-tuberculosis drugs, but they do respond to newer macrolides such as azithromycin and clarithromycin. In the case of a rapidly growing mycobacterium such as Mycobacterium abscessus (M. abscessus), antimicrobial treatment is further complicated by high levels of in vitro resistance. Thus, injectable antimicrobial drugs and longer durations of treatment are needed.5 Despite the use of these potentially toxic drugs, studies have reported treatment success rates of only 56.3% and 55.6%.6,7 In this context, surgical resection has been tried for localized M. abscessus lung disease.1,8,9 In the present work, we report a case involving a patient with M. abscessus lung disease, initially diagnosed as having M. intracellulare, who improved with sequential bilateral lung resection and anti-mycobacterial medications.
A 25-year-old woman arrived at our hospital citing fever and chills for 2 days. She also complained of a productive cough, night sweats, and general weakness for several weeks. She had been treated as if she had pulmonary tuberculosis (TB) three times previously, 17, 12, and 5 years earlier. Although the results from the mycobacterial studies of the first two episodes of TB were not available, the anti-TB treatments for the first and second episodes of TB had each continued for 6 months, and she reported that she took every medication as scheduled. Five years ago, she was diagnosed as having recurrent pulmonary TB based on an acid-fast staining of sputum. A mycobacterial culture was not performed. Treatment with isoniazid, rifampicin, ethambutol, and pyrazinamide was again started. However, the regimen was transiently changed to non-hepatotoxic drugs, including ethambutol, streptomycin, and cycloserine. She experienced several adverse drug effects: pancytopenia of rifampicin, ototoxicity of streptomycin, and neurotoxicity of cycloserine.
On admission to our hospital, her vitals were as follows: blood pressure, 110/50 mmHg; body temperature, 38.4℃; pulse rate, 126 bpm; and respiration rate, 20 breaths per minute. A physical examination revealed crackles in both lower lobes and inspiratory wheezing in the right middle lobe and the left upper lobe, and there was no scarring indicating a BCG vaccination on either arm. A chest roentgenogram showed air space consolidation in both lower lung zones with nodules and a right middle lobe collapse. A computerized tomography (CT) scan of the chest revealed bronchiectasis with a collapse of the right middle lobe and air space consolidation with cavity formation in the left lower lobe. In addition, in the right upper lobe and both lower lobes, there was branching linear opacity, revealing bronchogenic spread (Fig. 1). Laboratory findings included a white blood cell count of 20,500 cells/mm3 (segment form, 83.7%), hemoglobin of 11.6 g/dL, and platelet cell count of 241,000 cells/mm3. Arterial blood gas analysis performed while breathing without oxygen supplements revealed a pH of 7.39, partial pressure of oxygen of 82.2 mmHg, partial pressure of carbon dioxide of 37.8 mmHg, and oxygen saturation of 95.9%. Hepatic function was normal, except for a mildly elevated aspartate aminotransferase (AST) level of 62 U/L. Renal function was normal, and an human immunodeficiency virus (HIV) test was negative. Acid-fast staining of her sputa revealed numerous bacilli (1-9/10 HPF).
Assuming that she had drug-resistant pulmonary TB, we started a regimen that included isoniazid, ethambutol, levofloxacin, prothionamide, pyrazinamide, and p-aminosalicylic acid. We did not use rifampicin or aminoglycoside because of previous adverse effects. P-aminosalicylic acid was also discontinued within 2 weeks due to abdominal pain. Colonies of mycobacteria were identified in her sputa after 40 days of culture in the Lowenstein-Jensen medium. These bacilli were proved to be NTM, instead of M. tuberculosis complex, by a Gen-Probe test (Gen-Probe Inc., San Diego, CA, USA). These mycobacteria were identified as M. intracellulare by a previously reported polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method using cultured mycobacterial colonies at the Korean Institute of Tuberculosis.10 Under the diagnosis of M. intracellulare lung disease, the regimen was changed to clarithromycin, ethambutol, and moxifloxacin. Despite the regular intake of medication, her symptoms did not improve, and the results of smears for acid-fast bacilli were repeatedly positive (1-9/100 HPF). After 8 months of medication, M. abscessus was isolated instead of M. intracellulare (Table 1). A chest CT performed 1 year after the treatment began revealed minimal improvement (Fig. 1). Amikacin was added to the regimen after careful evaluation of her hearing function. However, she refused to try cefoxitin and imipenem because of possible leukopenia and high cost. We performed a bronchoscopic lavage to identify causative mycobacteria, and M. abscessus was isolated from the cultures of lavaged fluid from the right middle and left lower lobes.
Considering the poor symptomatic response to anti-mycobacterial medications and the persistence of a cavitary lesion, we decided to conduct a surgical resection. She underwent a video-assisted thoracoscopic right middle lobe lobectomy, right lower lobe wedge resection, and right upper lobe wedge resection. She was discharged 6 days after the operation without postoperative complication. On the second operation, which was conducted 1 month later, a video-assisted thoracoscopic left lower lobe basal segmentectomy and left upper lobe wedge resection were performed. She was discharged 11 days after the operation without postoperative complication. After the second operation, negative sputum conversion was achieved. At 11 months after the operation, the results of the smears for acid-fast bacilli were negative, and the amount of sputum had decreased. She was being kept on chemotherapy regimens with clarithromycin, ethambutol, and moxifloxacin without complaining of any respiratory symptoms (Table 1). Amikacin was discontinued after 12 months because of increasing tinnitus. A CT of the chest performed 9 months after the second operation revealed no new lesions (Fig. 1).
Approximately 40% of patients with M. abscessus lung disease have an underlying medical condition, such as previous mycobacterial infections, cystic fibrosis, lipoid pneumonia, lung transplantation, achalasia, or other conditions associated with recurrent vomiting.4,5,8 These patients often develop the disease at a younger age compared with patients without an underlying illness. In patients without associated medical conditions, the lung disease progresses very slowly, and the chest radiograph usually shows multilobar, patchy, reticulonodular or mixed interstitial-alveolar infiltrates with an upper lobe predominance.1
This patient was initially diagnosed as having M. intracellulare pulmonary disease; however, M. abscessus was isolated instead after the 8-month anti-mycobacterial treatment. Mycobacterium abscessus likely caused the patient's lung disease because it was isolated from the lavaged fluid from both lungs. In fact, alternative isolations of M. abscessus and M. intracellulare have been reported to occur in as many as 15% of patients with M. avium complex pulmonary diseases and 31% of patients with M. abscessus pulmonary disease.8,11 Clinicians should carefully decide which organism needs to be the target for treatment based on serial isolations of NTM.
The reported success rate of medical treatment in patients with M. abscessus pulmonary disease has been low. Griffith, et al.8 reported that only 10 of the 119 patients with M. abscessus were cured. Among these 10 patients, seven received antibiotic treatment followed by surgical resection of localized lesions, whereas only three were successfully treated with antibiotics alone.8 After the introduction of macrolides, the prognosis of medical treatment in patients with M. abscessus improved. In two Korean case series, nine out of 16 patients and five out of nine patients with M. abscessus were cured with medical treatment alone.6,7
Although surgical resection for localized mycobacterial disease can be associated with significant morbidity and mortality12 and the history of a underlying previous lung disease can limit the surgical approach,1 surgical resection can be used in select patients with localized M. abscessus lung disease. As far as we know, the patient in this report was the first case to undergo bilateral sequential lung resection for the treatment of M. abscessus lung disease. The fact that sputum conversion was obtained only after the second resection underscores the necessity for the bilateral resection in this patient. Based on our experience with this patient, we suggest that, if medical treatment fails, resection of a diseased lobe should be considered even in patients with bilateral lesions.
Figures and Tables
 | Fig. 1High-resolution computed tomography (HRCT) on admission showed the collapse with bronchiectasis of right middle lobe, cavitary lesion of left lower lobe, and multiple branching linear opacity on both lower lobes (A and B). Follow-up HRCT performed one year following the medical treatment revealed minimal improvement (C and D). No newly developed lesion was observed on HRCT taken eight months after bilateral lung resection (E and F). |
Table 1
Sequential Results of Acid Fast Smear, Mycobacterial Culture, and Identification of Sputa or Lavaged Fluid
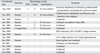
RML, right middle lobe; RUL, right upper lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
*Quantitation scale for Acid-Fast Bacillus smears and mycobacterial growth on agar plates followed diagnostic standards of American Thoracic Society.13
†M. abscessus was isolated from lavaged fluids right middle lobe and left lower lobe.
References
1. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156(2 Pt 2):S1–S25.
2. British Thoracic Society. Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Thorax. 2000. 55:210–218.
3. Yim JJ, Park YK, Lew WJ, Bai GH, Han SK, Shim YS. Mycobacterium kansasii pulmonary diseases in Korea. J Korean Med Sci. 2005. 20:957–960.

4. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005. 20:913–925.
5. Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002. 23:623–632.

6. Kim EK, Shim TS, Lim CM, Lee SD, Koh YS, Kim WS. Clinical manifestation of pulmonary infection due to rapidly growing nontuberculous mycobacteria. Tuberc Respir Dis. 2003. 54:283–294.

7. Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, et al. Treatment outcome of Mycobacterium abscessus pulmonary disease. Tuberc Respir Dis. 2003. 55:Suppl 2. 107.
8. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993. 147:1271–1278.

9. Kwon YS, Kwon OJ, Lee NY, Han J, Lee KS, Shim YM. Successful treatment of Mycobacterium abscessus lung disease with pneumonectomy combined with antibiotic therapy: a case report. Korean J Med. 2005. 69:424–427.
10. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000. 38:2966–2971.

11. Wallace RJ Jr, Zhang Y, Brown BA, Dawson D, Murphy DT, Wilson R, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998. 158:1235–1244.

12. Pomerantz M, Madsen L, Goble M, Iseman M. Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg. 1991. 52:1108–1111.

13. American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med. 2000. 161(4 Pt 1):1376–1395.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download