Abstract
A pathologic splenic rupture refers to a rupture without trauma. A splenic rupture as the initial manifestation of acute myeloid leukemia is extremely rare. In this study, we described a rare case of acute myeloid leukemia presenting principally as an acute abdomen due to a pathologic splenic rupture in a 35-year old male patient. We can assert that a pathologic splenic rupture in hematologic diseases is a potentially life-threatening complication, which necessitates immediate operative intervention. Any such patient complaining about left upper abdominal tenderness should be closely observed, and further diagnostic investigations (ultrasonograph of the abdomen, abdominal CT scan) should be initiated in order to rule out a splenic rupture. The oncologist should be aware of this rare initial presentation of acute myeloid leukemia (AML) M2, as the condition generally necessitates a prompt splenectomy.
Pathologic splenic ruptures re a rare and life-threatening complication of acute leukemia.1,2 A splenic rupture as the initial manifestation of acute myeloid leukemia is extremely rare, and only a few cases have been reported thus far in worldwide literature.3-5 It is an abdominal emergency which requires immediate diagnosis via an abdominal ultrasound and prompt splenectomy in order to ensure the survival of the patient. In this study, we describe a case of a pathologic splenic rupture as the initial manifestation of acute myeloid leukemia (AML) M2.
A 35-year old man was admitted to our hospital with a four-day history of periumbilical pain with dyspepsia and abdominal distension. The patient had no history of trauma. Upon physical examination, we noted generalized guarding with tenderness and rebound tenderness over the upper abdomen. The enhanced computed tomography (CT) scan of the abdomen/pelvis indicated multiple splenic lacerations, hepatosplenomegaly, and fluid collections in the perihepatic, perisplenic, both paracolic gutters, and pelvic cavity (Fig. 1).
The patient underwent a splenectomy. The operative finding was a massively enlarged spleen with multiple transverse lacerations. The microscopic findings of the spleen revealed diffuse infiltration of myeloperoxidase (MPO)-stained leukemic blasts (Fig. 2). His complete blood count (CBC) revealed the following: total white blood cell count of 21,099/µL, hemoglobin of 7.6 g/dL, and platelet count of 22,000/µL. On his disseminated intravascular coagulopathy (DIC) profile, prothrombin time (PT) 1.2, international normalized ratio (INR), activated partial thromboplastin time (aPTT) 27.6 seconds, fibrinogen 254 mg/dL, Fibrinogen degradation product (FDP) 75.8 µg/mL, and D-dimer > 16 µg/mL - these findings suggested DIC. He transfused 10 units of packed red blood cell (RBC) (320 mL) and 30 units of platelet concentrates (320 mL) for operation. The patient's peripheral blood smear showed neutrophilic leukocytosis with leukoerythroblastosis, microcytic anemia, and marked thrombocytopenia. The patient was subjected to a bone marrow biopsy examination. The bone marrow examination revealed a hypercellular marrow of approximately 70-80% cellularity. Normal myelopoiesis and erythropoiesis were both depressed, and no megakaryocytes were observed. We noted a marked proliferation of leukemic blasts, 48.6% of all nucleated cells, characterized by a high nuclear/cytoplasm ratio, fine nuclear chromatin, several distinct nucleoli, irregular nuclear and cytoplasmic membranes, and occasional cytoplasmic granules (Fig. 3). The leukemic blasts were shown to be positive for CD13, CD33, and CD56, and were also positive for MPO and PAS. Conventional cytogenetic analysis on the G-banded metaphases revealed the following: 46,XY,der(7)t(7;9) (p22;p21)inv(9)(p12q 13),der(9)t(7;9)inv(9). The results revealed AML of M2 subtype.
Ten days after the splenectomy, the patient was treated with an induction of chemotherapy using cytosine-arabinoside (100 mg/m2, days 1-7) and idarubicin (12 mg/m2, days 1-3). He was subsequently treated with two cycles of consolidation chemotherapy using cytosine-arabinoside (3 g/m2, days 1-3) and idarubicin (12 mg/m2, days 1-2). The patient achieved complete remission, but then relapsed after 5 months. Reinduction chemotherapy using a FLAG-IDA regimen6 - fludarabine (30 mg/m2, days 1-5), cytosine-arabinoside (2 g/m2, days 1-7), and idarubicin (10 mg/m2, day 1-3) failed to induce remission. The patient was then treated with two more cycles of reinduction chemotherapy, but did not achieve complete remission. He was in a refractory AML state and suffered from intra-abdominal abscess, abdominal pain, and severe hepatomegaly. Due to his low performance status, further chemotherapy could not be administered, and the patient was treated with supportive care.
A pathologic splenic rupture refers to rupture without trauma. A normal spleen does not rupture in the absence of trauma. Therefore, any "spontaneous" rupture should prompt a search by the clinician for any underlying splenic pathology.7
The majority of clinical symptoms of a splenic rupture include abdominal pain, abdominal tenderness and rigidity, hypotension, tachycardia, nausea and vomiting, dizziness, and syncope.1 The diagnosis of this condition is based on clinical signs and confirmatory diagnostic tests. Using ultrasonography or a CT and peritoneal aspirations of fresh blood may facilitate the diagnosis of apathological splenic rupture. CT imaging of the abdomen, although it is unsuitable for hemodynamically unstable patients, is currently the imaging method of choice. It is relatively rapid and accurate, and presents minimal risk to the patient.8
The exact etiology of a splenic rupture in hemotologic malignancies remains to be firmly established. The possible mechanisms suggested thus far include splenic enlargement, leukemic blasts infiltration of the splenic capsule, splenic infarction resulting in capsular hemorrhage, and leukemia-associated coagulopathy.8 Male patients, patients above 20 years of age, patients displaying splenomegaly, and those who have recently undergone cytoreductive chemotherapy appear to be at higher risk of suffering a pathologic rupture.1
Splenectomy is constituted the treatment of choice for adults with leukemia and a pathologic splenic rupture. Splenorrhaphy and partial splenectomy are generally thought to play no role in the management of pathologic splenic ruptures in leukemia patients. Without surgical intervention, the mortality rate reported in this population approaches 100%.2 Therefore, early surgical intervention and appropriate hemoderivative support is most important.
Figures and Tables
Fig. 1
Computed tomography (CT) image of the upper abdomen demonstrates multiple splenic lacerations evidenced by the irregular, nonhomogeneous, low density of splenic body (red arrows).
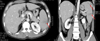
Fig. 2
(A) The spleen shows blood clots attached to the ruptured capsule (H-E stain, ×20). (B) The spleen evidences diffuse invasion by immature myeloid cells (H-E stain, ×200). (C) The infiltrates are composed principally of myeloperoxidase-positive immature myeloid cells (Myeloperoxidase stain, ×400).

References
1. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996. 73:297–302.

2. Bernat S, García Boyero R, Guinot M, López F, Gozalbo T, Cañ igral G. Pathologic rupture of the spleen as the initial manifestation in acute lymphoblastic leukemia. Haematologica. 1998. 83:760–761.
3. Görg C, Barth P, Weide R, Schwerk WB. Spontaneous splenic rupture in acute myeloid leukemia: sonographic follow-up study. Bildgebung. 1994. 61:37–39.
4. Wong P, Takabayashi K, Sugiura Y, Asai T, Itoh K, Yoshida S, et al. Splenic rupture in acute megakaryoblastic leukemia. Jpn J Med. 1987. 26:234–236.
5. Rajagopal A, Ramasamy R, Martin J, Kumar P. Acute myeloid leukemia presenting as splenic rupture. J Assoc Physicians India. 2002. 50:1435–1437.
6. Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, Rizzi R, et al. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003. 82:231–235.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download