INTRODUCTION
Acanthamoeba keratitis (AK), a sight-threatening progressive corneal disease, is commonly caused by ubiquitous, pathogenic, free-living species, which are widely distributed in the environment.1,2 Acanthamoeba exists in two stages as both mobile trophozoites and dormant cysts. The trophozoites can cause radial neuritis, resulting in severe pain, and treatment is difficult because of the resistance of the cyst, which has a bilaminated cellulose wall, to most antimicrobial agents.3-6 Many patients have to rely on keratoplasty that is largely limited by the availability of corneal donors, rejection response after operation, and recurrence caused by latent cysts.4 Since 1973 when the first case was reported, AK has become an important blinding ocular infection worldwide. There have been reports of keratitis, uveitis and endophthalmitis.5-7 In recent years, the popularity of human eye contact lenses has increased the number of AK infections. Corneal injury is considered to be an another important risk factor for AK. A three-year study at a tertiary eye care referral center in South India indicated that the incidence of AK amongst the corneal ulcer patients was 1% and it was mainly due to corneal injury from mud.8 A large survey of Chinese patients in 2002 showed that Acanthamoeba positive cases accounted for 2.4% of all corneal infections.9
The development of an animal model of AK is essential for detailed study of molecular biology, pathology and immunology of AK and for control of in vivo testing of new pharmacologic agents.10,11 As corneal Acanthamoeba infection is still relatively rare compared to the infection by other pathogens, it is important to use the proper kind of animal and the most effective method in order to draw correct conclusions. In previous studies, pigs, rabbits, and Chinese hamsters have been used to produce AK.12-15 Rats and mice are the most widely used animals, since they are easy and cheap to keep in large numbers and there are rich resources of molecular reagents such as antibodies for these animals. In this study, we established rat and mouse models of AK in 3 different ways, examined clinical manifestations of these models, and evaluated the advantages, limits and adaptation range of each experimental method, to hopefully provide a better basis for research into AK.
MATERIALS AND METHODS
Culture of Acanthamoeba and preparation of stimulating solution
Acanthamoeba spp., clinically isolated and characterized as genotype T4, was provided by the Beijing Eye Institute. The parasites were grown anemically in 25 cm2 canted-neck tissue culture flasks containing 5 mL of PYG medium (Peptone-Yeast-Glucose) at 30℃ until 95% was in trophozoite form after one week of culture. Approximately 1×106 cells of Acanthamoeba were harvested from each flask and washed three times with phosphate-buffered saline (PBS) by centrifuging at 500 g for 7 minutes. Each precipitate was suspended in 1 mL of PBS to be used as the stimulating solution.
Preparation of contact lenses
Sixty soft contact lenses (1 Day ACUVUE, Johnson & Johnson, Vision Care, Shanghai, China) were bought from optical shops. Thirty bigger lenses of suitable size for rats (inner diameter of 4 mm) and thirty smaller ones for mice (inner dismeter of 2 mm) were made from these contact lenses by a sterile corneal trephine (66vision Tech Co., Ltd, Suzhou, China) under an operating microscope (66vision Tech Co., Ltd, Suzhou, China). All procedures were undertaken in a bacteria - free environment. Then, all the contact lenses were washed 3 times and incubated overnight with multi-functional solution. Fifteen bigger lenses and fifteen smaller ones were washed with PBS and incubated with Acanthamoeba solution for 1 hour for the parasites to attach to the lenses as contaminated lenses, whereas the other lenses were washed with PBS to be used as controls.
Animals
Forty-five Wistar rats, weighing 280 g to 300 g, and 45 Kunming mice, weighing 28-32 g, regardless of their gender were obtained from the Animal Supply Centre of Shandong University. Animal care and treatment in this investigation conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Slit lamp examination prior to the experiments was used to exclude any injury to the cornea. The rats and mice were randomly divided into groups A, B, and C with 15 rats and 15 mice in each group. Five rats or mice in each group were used for histopathological investigations and the other ten were used for clinical evaluations. Anti-microbial chloromycetin eye drops (Lukang Co., Shandong, China) were applied frequently one day before the inoculation to prevent bacterial co-infection.
Model of invasive keratitis
The animal models of AK were established in the following 3 ways. Each procedure was performed under an operation microscope.
Treatment of animals in group A
The animal corneas in group A were scratched and challenged with Acanthamoeba: The rats and mice in group A were generally anesthetized with 10% chloral hydrate (3 mL/kg) injected peritoneally. Corneal anesthesia was obtained with topical 0.4% Oxybuprocaine hydrochloride eye drops (Santen Pharmaceutical (China) Co., Ltd., Suzhou, China). After routine disinfection, the corneas of both eyes were scratched three times vertically and three times horizontally with a sterile 30-gauge syringe needle and the stimulating solution was applied to the scarified corneas of the right eye while PBS was used on the left eye as control. Then, the eyelids of all the right eyes were sutured to guarantee full contact between Acanthamoeba and the injured corneas. Topical application of erythromycin ophthalmic ointment (Lukang Co., Ltd., Shandong, China) was done for anti-bacterial purposes. Twenty-four hours later, the stitches were removed and the corneas were monitored by slit lamp examination.
Treatment of animals in group B
The animal corneas in group B were scratched and covered with eye contact lenses. They were anesthetized, disinfected and scratched just as those in group A. Acanthamoeba solution was applied to the right eyes of all the animals in group B before the contact lenses were inserted. The left eyes, used as control, were covered with contact lenses pretreated with sterile Acanthamoeba-free PBS. Erythromycin ophthalmic ointment was used for anti-bacterial purposes. The contact lenses were removed after 24 hours and slit lamp examination was used to monitor the animals.
Treatment of animals in group C
Acanthamoeba solution was intrastromally injected into the corneas of group C animal. The animals were anaesthetized and disinfected. A solution of 1×106 cells/mL Acanthamoeba was injected into the stroma of the right eye with a micro liter syringe and 30 G needle, using 1 µL for mice and 2 µL for rats. As a control, the left eye of each animal received a mock inoculation of PBS.
Methods for confirmation of Acanthamoeba infection
Microscopic examination of 10% potassium hydroxide wet mounts of corneal scraping was performed to observe Acanthamoeba trophozoites. The tissues from corneal scraping were examined under a light microscope and incubated with PYG medium containing non-nutritive agar to confirm the models and to exclude co-infection with bacteria or fungi.
Clinical evaluation
On days 1, 3, 7, 13 and 21 post infection, the animals were monitored by slit lamp examination. A grade of 0 to 4 was assigned to each, based on the following three criteria: area of opacity, density of opacity and surface regularity (Table 1). A normal untreated cornea was given a score of 0 in each category and thus had a total score of 0. The scores from all three categories were tallied at 1, 3, 6, and 10 days post infection for each eye to yield a possible total score, ranging from 0 to 12. A total score of 5 or less was categorized as mild infection, a total score of 6 to 9 was considered moderate, and a total score of more than 9 was severe. At the end of the experiments, the other corneas were collected and stored at -80℃ for future analysis.
Pathological analysis of AK
On days 0, 1, 3, 7, 13, and 21 post infection, infected corneas of 5 animals in each group were harvested and paraffin sections were made for Hematoxylin-Eosin (HE) staining to observe pathological process of AK in these animal models under a microscope. At the end of the experiments, the other corneas were collected for future analysis.
RESULTS
Confirmation of Acanthamoeba infection
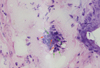
In the present study, laboratory tests such as examination of 10% potassium hydroxide wet mount of corneal scraping tissues, HE staining of the corneal sections and incubation of corneal scrapings were used to confirm AK and to exclude co-infections. The tests were negative for bacteria and fungi, and there were no colonies of bacteria or fungi formed in the culture medium. Typical cysts were observed on 10% potassium hydroxide wet mounts of corneal scraping tissues and HE staining of corneal sections under a duplex inverted microscope (Figs. 1 and 2). Active trophozoites grew in PYG medium with corneal tissues after 10 days of incubation.
Clinical evaluation of AK models
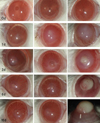
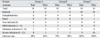
The clinical features of AK were recorded, scored and graded with the aid of a dissecting microscope and slit lamp examination at 0, 1, 3, 7, 13, and 21 days post infection (Table 2). As rats and mice models developed similar clinical courses, pictures of rat corneas only in group B are presented in Fig. 3. Four rats and 6 mice in group A, 7 rats and 8 mice in group B, and 10 rats and 10 mice in group C developed Acanthamoeba keratitis. In group A, no severe inflammation was observed in any of the rats or mice. The corneas of 6 rats and 4 mice recovered within just 24 hours after the treatment. In group B, slight corneal edema and then complete recovery were observed in 3 rats and 2 mice. The infected corneas developed the typical ring ulcer of Acanthamoeba keratitis. In group C, most animals developed severe inflammations with ring ulcers and obvious stroma abscesses. Two mice developed even severe endophthalmitis and one of them died 13 days after the treatment. From the 13th day to the 21st day after the treatment, angiogenesis appeared in all three groups and the infections in groups A and B were gradually alleviated with decreasing ulcers and repair of scarring, but the inflammation in group C was aggravated with increasing abscesses and the infection spreading to involve nearly the whole cornea. After one month, most of the inflammation was alleviated or healed.
Pathology of AK in the animal models
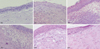
The corneas of the animal models were harvested and treated for pathological analysis. Fig. 4 shows the pathological process of AK in rats of group B. The picture of "0d" is the corneal section of normal rats in which the epithelium and stroma are well-defined, the stroma fibers are arranged regularly, and there are no inflammatory cells infiltrated. In the corneal sections of the AK animal models, the normal structure was destroyed. On the 1st day post infection, a few inflammatory cells were infiltrated just beneath the corneal epithelia. On day 3 post infection, more inflammatory cells were infiltrated in the cornea. On the day 7, most of the epithelia and stroma were infiltrated by a large number of inflammatory cells and the structure of the epithelia was unorganized. Tissue necrosis could be observed and the whole structure of the cornea was destroyed. On the 13th day, the amount of inflammatory cells decreased and neovascularization could be observed. Twenty-one days post infection, the corneal stroma was full of irregular collagen fibers, demonstrating scar healing in the cornea.
Statistical analysis of the three different methods
To evaluate the effect of the three different treatment methods, the infection rate of each group was analyzed and the results are listed in Table 2.
We used the SPSS12.0 program to do the statistical analysis. The results suggested significant differences among the three groups (p < 0.01).
DISCUSSION
In the present study, AK models were established in rats and mice using 3 different techniques without the need for concomitant immunosuppression by corticosteroids or coinoculation with bacteria. The methods to produce AK models were evaluated clinically, histologically, and statistically for 10 days after inoculation.
Typically, AK has a progressive course with intense discomfort and stromal infiltration. It is often initially misdiagnosed as herpes simplex keratitis or adenoviral keratitis. Early clinical signs of AK include epithelial irregularities, opacities, microerosions, microcystic edema, and patchy anterior stromal infiltrates. Late in the disease course (prolonged infection), limbal hyperemia, edema, and ring infiltrate can develop. Ring infiltrate is considered to be the representative sign of AK.
It is crucial to choose proper kind of animals for in vivo experiments.10 In some early studies on corneal disease, mice, rats, New Zealand rabbits, hamsters and pigs have been all used.11,12 We chose mice and rats instead of the others to establish the models of AK because they are easier to operate, less expensive to keep in large numbers and specific and abundant commercial anti-mice or anti-rat antibodies are available at present which is beneficial to further research.13,16 The present study compared rats and mice and found that it was easier for mice to develop AK. However, we found during the experiments that mice had a certain death rate even with surface infection. We repeated the same experiment several times and still found that some deaths were inevitable. The body size, immune response levels, and limited safe anesthetic dosage range all gave rise to an increased death risk for mice. Rats are superior models not only for their suitable size and immune response, but also because the size of their eyes makes controlled surgical procedures easier.
Acanthamoeba keratitis has a lower incidence than keratitis caused by other pathogens.9 The treatment processes of groups A and B in this study were simulations of corneal trauma and contact lens-related keratitis and they are also suitable for use in studies of the mechanism of antigencontact, signal transduction and intracellular signal cascades of corneal epithelial cells. In our previous studies, we found that Acanthamoeba could successfully attach to the lenses after one hour of co-incubation. Therefore, we pretreated the lenses with Acanthamoeba solution for 1 hour to produce infected lenses. The intrastromal injection of Acanthamoeba in group C triggered a rapid response and developed typical stromal ring ulcers in animal corneas. Two mice in this group developed even severe endophthalmitis besides typical Acanthamoeba keratitis. Nevertheless, it is noteworthy that this kind of treatment would not be a natural route for Acanthamoeba, which enters mainly from the surface. If we are to do research on the molecular biology, pathological analysis, or biological and immunological studies of corneal stromal cells during AK, or controlled in vivo testing of new pharmacologic agents against AK, this method has great advantages in improving the efficiency of infection in animal models.
In the present study, some rats and mice in group A and B were not infected. They all had wound healing in 24 hours and the corneas recovered completely after a short period of cloudiness. The corneas were then harvested for pathological analysis, and no Acanthamoeba cysts or trophozoites were found in the corneal sections. It was concluded that the corneas construct a first line of defense against pathogens and they are capable of defending the eyes from the invasion and diffusion of Acanthamoeba. In group C, all the animals were infected, which showed that intrastromal injection of Acanthamoeba was the most effective way to produce AK animal models. What is more interesting in the present study was that most of the animal models could heal themselves without treatment, which is far different from human beings. The same phenomenon has been observed in the rat models of fungal keratitis in our laboratory [unpublished]. This suggests that rats and mice have a more effective immune system than human beings do, but no definite research finding can yet explain the differences. Further studies should be done on this subject.
In conclusion, the present study shows that rats are preferred as keratitis models over mice because they have a low death rate and larger corneas for inoculation. The intrastromal injection of Acanthamoeba was the most effective way to establish the rat or mice models of AK. After corneal scratching, the infection developed, similarly to natural infections, however, this method had the lowest infection rate. Corneal scratching and exposure to Acanthamoeba via wearing contact lenses simulated contact lens-related AK best, and had a moderate infection rate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download