Abstract
Purpose
Combination antibiotic treatment is preferred in nosocomial infections caused by Pseudomonas aeruginosa (P. aeruginosa). In vitro synergism tests were used to choose the combinations which might be used in clinic. The aim of this study was to investigate the synergistic efficacy of synergistic antibiotic combinations in multidrug resistant P. aeruginosa strains.
Materials and Methods
Synergistic efficacies of ceftazidime-tobramycin, piperacillin/tazobactam-tobramycin, imipenem-tobramycin, imipenem-isepamycin, imipenem-ciprofloxacin and ciprofloxacin-tobramycin combinations were investigated by checkerboard technique in 12 multiple-resistant and 13 susceptible P. aeruginosa strains.
Results
The ratios of synergy were observed in ceftazidime-tobramycin and piperacillin/tazobactam-tobramycin combinations as 67%, and 50%, respectively, in resistant strains, whereas synergy was not detected in other combinations. The ratios of synergy were observed in ceftazidime-tobramycin, piperacillin/tazobactam-tobramycin, imipenem-tobramycin, imipenem-ciprofloxacin and imipenem-isepamycin combinations as 31%, 46%, 15%, 8%, 8%, and respectively, in susceptible strains, whereas synergy was not detected in ciprofloxacin-tobramycin combination. Antagonism was not observed in any of the combinations.
Conclusion
Although the synergistic ratios were high in combinations with ceftazidime or piperacillin/tazobactam and tobramycin, the concentrations in these combinations could not usually reach clinically available levels. Thus, the solution of the problems caused by multiple resistant P. aeruginosa should be based on the prevention of the development of resistance and spread of the causative agent between patients.
Hospital infections are important health problems in all over the world, because of their high morbidity and mortality, and prolonging the time of hospitalization and increasing the cost of treatment. Pseudomonas aeruginosa (P. aeruginosa) is recognised as one of the leading causes of severe hospital-acquired infections. P. aeruginosa exhibits high-level resistance to many antimicrobials, and resistance can develop during therapy. Combination antibiotic treatment is preferred to provide larger spectrum antimicrobial effect and to prevent the rapid emergence of resistance in nosocomial infections caused by P. aeruginosa. Combinations usually comprise an anti-pseudomonal beta-lactam and an aminoglycoside or a fluoroquinolone.1
The aim of this study was to determine the in vitro effects of some antimicrobial drug combinations on multidrug resistant P. aeruginosa strains and compare them with the susceptible P.aeruginosa strains.
Study isolates were chosen from nosocomial P. aeruginosa isolates collected from January 1996 through August 2000 from the Clinical Microbiology Laboratory of Trakya University Hospital in Edirne, Turkey. A total of 25 non-duplicate isolates were included in the study. Twelve of them were multidrug resistant and 13 of them were susceptible to antimicrobial agents. Strains resistant to ureidopenicillin, third generation cephalosporin, azteronam, quinolone, carbapenem and at least two aminoglicosides were accepted as multidrug resistant. The isolates were cultured from urine (7 isolates), lower respiratory tract (6 isolates), blood (6 isolates) and skin-soft tissues (6 isolates). P. aeruginosa ATCC 27853 was included as a quality control strain. The isolates were stored at -70℃ and studied after being subcultured twice on blood and EMB agar (Diomed, Istanbul, Turkey). All strains were identified by the conventional methods and confirmed by API 20 NE (Bio Mérieux, Marcy I'Etoile, France).
Antibiotic powders were obtained from the manufacturers as follows: Ceftazidime (Glaxo-Wellcome, Turkey), piperacillin and tazobactam (Wyeth, Istanbul, Turkey), imipenem (Merck-Sharp and Dohme, Istanbul, Turkey), ciprofloxacin (Bayer, Istanbul, Turkey), tobramycin (Eczaclba l, Istanbul, Turkey) and isepamycin (Schering-Plough). Stock solutions were prepared using sterile water and stored at -70℃ until use, with the exception of imipenem which was prepared immediately prior to use.
Disc diffusion test was performed, by using piperacillin (100 µg), ceftazidime (30 µg), piperacillin-tazobactam (100 + 10 µg), sulbactam/sefoperazon (30 + 75 µg), imipenem (10 µg), ciprofloxacin (5 µg), amikacin (30 µg), gentamicin (120 µg), netilmicin (30 µg), tobramicin (10 µg) (Oxoid, Hampshire, England) and isepamycin (30 µg) (Mast, Merseyside, England) discs, and the result was interpreted according to Clinical and Laboratory Standards Institute (CLSI) methodology.2,3 Breakpoints for isepamycin were those recommended by Barry, et al.4 and breakpoints for cefoperazone-sulbactam were those recommended by the manufacturer.
The minimum inhibitory and bactericidal concentrations (MICs and MBCs) of ceftazidime, piperacillin/tazobactam, imipenem, ciprofloxacin, tobramycin and isepamycin were determined by broth microdilution method as described by CLSI, and CLSI criteria were used in the interpretations of the results.4,5
Serial two-fold dilutions, ranging from 0.125 to 256 µg/mL, for each antibiotics were prepared in cation-adjusted Mueller Hinton broth (CAMHB). The inoculum was prepared with 2-3 h broth culture of each isolate, adjusted to a turbidity equivalent to 0.5 McFarland Standard and diluted in CAMHB to give a final concentration of 5×105 CFU/mL in the test tray. Trays were covered and then incubated for 16-20 h in ambient air at 37℃. MIC was defined as the lowest concentration of antibiotic to completely inhibit visible growth.
MBCs were determined by removing 10 µL samples from each well, demonstrating no visible growth, and plated onto separate blood agar plates. After an incubation at 37℃ for 16-20 h, colonies were counted. MBC was defined as the lowest concentration of antibiotic to have at least 99.9% killing of the initial inoculum.6
In-vitro interactions of ceftazidime-tobramycin, piperacillin/tazobactam-tobramycin, imipenem-tobramycin, imipenem-isepamycin, imipenem-ciprofloxacin and ciprofloxacin-tobramycin combinations were investigated by microdilution checkerboard technique using 96-well microtiter plates for each combination.
Serial two-fold dilutions of the antimicrobial agents in CAMHB were placed alone or in combination in wells and inoculated with an appropriate bacterial inoculum so that each well contained approximately 4-5×104 CFU/mL. After incubation at 37℃ for 16-20 h, the MIC was considered as the well containing the lowest concentrations of the two drugs in which no visible growth was observed. Concentrations of each antimicrobial which were tested in combination were between 1/4× MIC and 2× MIC. Growth and sterility controls were also included in each plate.7,8
The fractional inhibitory concentrations (FICs) for each isolate were calculated as the MIC of drug A or B in combination/the MIC of drug A or B alone. The FIC index (ΣFIC) was calculated by summing the individual FICs obtained from the two antimicrobial agents. Synergy was defined as a FIC index of ≤ 0.5, additivity (indifference) by a FIC index of > 0.5 to ≤ 4 and antagonism by a FIC index of > 4. The lowest ΣFIC value calculated in the same plate for one strain and one combination was determined as ΣFICmin, and the highest ΣFIC value was determined as ΣFICmax.
Resistance patterns of the 12 multidrug resistant strains are shown in Table 1. Thirteen strains which served as the control group were susceptible to all of these antimicrobials.
The results of MIC and MBC testing of the 12 resistant and 13 susceptible strains are presented in Table 2. The MBC values were generally equal or one to three times greater than those of MIC.
The ratios of synergy were observed in ceftazidime plus tobramycin and piperacillin/tazobactam plus tobramycin combinations as 67% and 50%, respectively, in resistant strains, and synergy was not detected in other combinations. The ratios of synergy were observed in ceftazidime-tobramycin, piperacillin/tazobactam-tobramycin, imipenem-tobramycin, imipenem-ciprofloxacin and imipenem-isepamycin combinations as 31%, 46%, 15%, 8% and 8%, respectively, in susceptible strains and synergy was not detected in ciprofloxacin-tobramycin combination. Antagonism was not observed in any of the combinations. The results of the checkerboard synergy analysis are shown in Table 3.
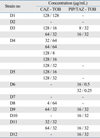
Antibiotic concentrations at which synergistic interactions were observed in multidrug resistant strains are shown in Table 4. Concentrations of ceftazdime and piperacillin/tazobactam which provided synergy in resisant strains were within clinically achievable limits (under 160 µg/mL for ceftazdime and under 264.4-368 µg/mL for piperacillin/tazobactam). However, only a few concentrations of tobramycin were in the range of clinically achievable limits (under 16-24 µg/mL).
Multidrug resistance in P. aeruginosa is a serious growing problem all over the world. Combination therapy is recommended for the treatment of P. aeruginosa infections in order to ensure synergistic action and decrease the risk of development of resistance. In vitro synergy tests, including checkerboard and time-kill methods, are used to determine the activity of antimicrobial combinations. In spite of some limitations, the checkerboard technique is simple to perform and remains to be a widely used technique to assess antimicrobial combinations.8 Comparison of results from different studies is difficult due to variations in microbiology test materials, methods and synergy definitions.9
Beta-lactam and aminoglycoside combinations are the combinations most frequent used for the treatment of P. aeruginosa infections.2,10 Synergistic interactions of these combinations have been reported in many studies.11-26 In the present study, we detected synergy only in ceftazidime-tobramycin (67%) and piperacillin/tazobactam-tobramycin (50%) combinations in resistant strains, and in ceftazidime-tobramycin (31%), piperacillin/tazobactam-tobramycin (46%), imipenem-tobramycin (15%) and imipenem-isepamycin (8%) combinations in susceptible strains. No statistically significant differences in synergy rates were found between resistant and susceptible strains. However, Chan reported more synergy in susceptible strains than in resistant strains.13 On the other hand, several investigators demonstrated that synergy rates are not affected by individual resistance rates of the drugs in combination, similar to our study.14,15,17,18
Weiss and Lapointe11 detected less synergy in imipenem-tobramycin combination than the other beta-lactam combinations. In our study, synergy was not detected in imipenem-tobramycin and imipenem-isepamycin combinations in resistant strains and low synergy rates (15% and 8%, respectively) were observed in susceptible strains. All of the resistant strains except two (D2 and D6) were resistant to tobramycin. In D2, synergy was not detected in any combinations and, in D6, synergy was detected only in piperacillin/tazobactam-tobramycin combination. Only two resistant strains (D7 and D8) were resistant to isepamycin. Synergy with imipenem-isepamycin combination was observed only in one susceptible strain. It is reported that synergy rates are high with cephalosporin-isepamycin combinations, whereas imipenem-isepamycin combination is less effective.24 We considered that imipenem resistance might have affected the occurences of synergy. However, low resistance rates of isepamycin did not induce synergy.
There are many reports in the literature on synergism with several rates observed with quinolone and beta-lactam antibiotics.26-30 In our study, synergy was not demonstrated in resistant strains with imipenem-ciprofloxacin combination while synergy was observed in 8% of the susceptible strains. Combination of aminoglycosides with fluoroquinolones rarely shows synergy.19,28,30,31 We observed additive effect with ciprofloxacin-tobramycin combination in all of the strains.
Furthermore, none of the antimicrobial combinations tested in the current study demonstrated antagonism against any of the isolates tested.
Beta lactam-aminoglycoside combinations were shown to be the most effective combinations against P. aeruginosa. However, clinically achievable plasma concentrations at customary dosages might be important limitation in the use of these combinations for providing synergic effects. Mean peak plasma concentration of ceftazidime (2 g) intravenously every 6-8 hours has been reported to be 160 µg/mL.32 This concentration is over the concentrations of ceftazdime which provided synergy in our study. In beta-lactam antibiotics, drug concentrations which is over the MIC values must be maintained constant to reach bactericidal effect. For this reason, it is not possible to continuously reach this concentration, because of short half-life of the drug (two hours). Mean peak plasma concentrations of tobramycin (5.1 mg/kg and 7 mg/kg every 8 hours) daily dose have been reported to be 4-10 µg/mL and 16-24 µg/mL, respectively.33 Although tobramycin concentrations in which synergy was observed were with clinically achievable limits in ceftazidime-tobramycin combination in some resistant strains, it is highly possible that this combination is ineffective because ceftazidime concentrations were not in clinically achievable limits. Similarly, mean peak plasma concentration of piperacillin/tazobactam (3.375 g every 6 hours) has been reported to be 264.4-368 µg/mL34 and concentrations in which synergy was observed were lower than this value. However, piperacillin/tazobactam-tobramycin combination can not be considered as clinically effective in resistant strains except for D6, because tobramycin concentrations were not with clinically achievable limits. On the other hand, both ceftazidime-tobramycin and piperacillin/tazobactam-tobramycin combinations can be effective in urinary tract infections because the drugs are excreted from the body through the kidney unchanged with higher concentrations than serum. All of the synergistic interactions in susceptible strains were with clinically achievable concentrations (data not shown).
In conclusion, the variability of the results obtained in several studies may be due to differences in methodology, definitions of synergy and choice of the strains. Furthermore, there may not be a correlation between in vitro synergy and clinical efficacy. Therefore, additional in vivo studies to assess clinical efficacy of combinations are warranted. Moreover, antibiotic concentrations in synergistic combinations may not always reach clinically available levels. Thus, the solution of the problems caused by multiple resistant P. aeruginosa should be based on the prevention of development of resistance and spread of causative agents between patients.
Figures and Tables
Table 1
Resistance Patterns of the 12 Multidrug Resistant Strains according to Disk Diffusion Testing

Dn, Multidrug resistant strains; IPM, imipenem; PIP, piperacillin; PIP/TAZ, piperacillin/tazobactam; ATM, aztreonam; OFX, ofloxacin; CIP, ciprofloxacin; CAZ ceftazidime; CFP, cefoperazone; CTX, cefotaxime; CRO, ceftriaxone; AK, amikacin; GM, gentamicin; TOB, tobramycin; NET, netilmicin; ISP, isepamycin; R, resistant; S, susceptible; I, intermediate (the intermediate strains are accepted as resistant).
*Also evaluated by MIC determination.
ACKNOWLEDGMENTS
A part of the study has been reported as a poster presentation in "XII. International Congress of Bacteriology and Applied Microbiology, 5-9 August 2008, Istanbul".
References
1. Pier GB, Ramphal R. Mandell GL, Bennet JE, Dolin R, editors. Pseudomonas aeruginosa. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 2005. Volume 2:6 th edition. Philadelphia: Churchill Livingstone;2587–2615.
2. Clinical and laboratory standards institute. Performance standards for antimicrobial disk susceptibility tests. 2006. CLSI Document M2-A9 9th edition. Wayne, PA, USA: CLSI.
3. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. 2006. CLSI Document M100-S16 16th edition. Wayne, PA, USA: CLSI.
4. Barry AL, Thornsberry C, Jones RN, Gerlach EH. Interpretive standards for disk susceptibility tests with Sch21420 and amikacin. Antimicrob Agent Chemother. 1980. 18:616–621.

5. Clinical and laboratory standards institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2003. CLSI Document M7A6 6th edition. Wayne, PA, USA: CLSI.
6. Moody JA, Knapp C. Isenber HD, editor. Tests to assess bactericidal acivity. Clincal microbiology procedures handbook. 2004. Volume 2:2nd edition. Washington: ASM Press;5.10.1.1–5.10.1.17.
7. Moody J. Isenber HD, editor. Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. Clincal microbiology procedures handbook. 2004. Volume 2:2nd edition. Washington: ASM Press;5.12.1–5.12.23.
8. Eliopoulos GM, Moellering RC JR. Lorian V, editor. Antimicrobial Combinations. Antibiotics in Laboratory Medicine. 1996. 4 th edition. Baltimore: Williams & Wilkins;330–396.
9. Sader HS, Huynh HK, Jones RN. Contemporary in vitro synergy rates for aztreonam combined with newer fluoroquinolones and beta-lactams tested against gram-negative bacilli. Diagn Microbiol Infect Dis. 2003. 47:547–550.
10. Giamarellou H. Aminoglycosides plus beta-lactams against gram-negative organisms. Evaluation of in vitro synergy and chemical interactions. Am J Med. 1986. 80:126–137.

11. Weiss K, Lapointe JR. Routine susceptibility testing of four antibiotic combinations for improvement of laboratory guide to therapy of cystic fibrosis infections caused by Pseudomonas aeruginosa. Antimicrob Agent Chemother. 1995. 39:2411–2414.

12. Owens RC JR, Banevicius MA, Nicolau DP, Nightingale CH, Quintillani R. In vitro synergistic activities of tobramycine and selected beta-lactams against 75 gram-negative clinical isolates. Antimicrob Agent Chemother. 1997. 41:2586–2588.

13. Chan EL, Zabransky RJ. Determination of synergy by two methods with eight antimicrobial combinations against tobramycin-susceptible and tobramycin-resistant strains of Pseudomonas. Diagn Microbiol Infect Dis. 1987. 6:157–164.

14. Baltch AL, Bassey C, Hammer MC, Smith RP, Conroy JV, Michelsen PB. Synergy with cefsulodin or piperacillin and three aminoglycosides or aztreonam against aminoglycoside resistant strains of Pseudomonas aeruginosa. J Antimicrob Chemother. 1991. 27:801–808.
15. Perea EJ, Nogales MC, Aznar J, Martin E, Iglesias MC. Synergy between cefotaxime, cefsulodin, azlocillin, mezlocillin and aminoglycosides against carbenicillin resistant or sensitive Pseudomonas aeruginosa. J Antimicrob Chemother. 1980. 6:471–477.
16. Oie S, Sawa A, Kamiya A, Mizuno H. In vitro effects of a combination of antipseudomonal antibiotics against multi-drug resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 1999. 44:689–691.

17. Giamarellos-Bourboulis EJ, Grecka P, Giamarellou H. Comparative in vitro interactions of ceftazidime, meropenem and imipenem with amikacin on multiresistant Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1997. 29:81–86.

18. Clark RB, Pakiz CB, Hostetter MK. Synergistic activity of aminoglycoside-beta-lactam combinations against Pseudomonas aeruginosa with an unusual aminoglycoside antibiogram. Med Microbiol Immunol. 1990. 179:77–86.
19. Gerçeker AA, Gürler B. In vitro activities of various antibiotics, alone and in combination with amikacin against Pseudomonas aeruginosa. J Antimicrob Chemother. 1995. 36:707–711.
20. Bustamante CI, Drusano GL, Wharton RC, Wade JC. Synergism of the combinations of imipenem plus ciprofloxacin and imipenem plus amikacin against Pseudomonas aeruginosa and other bacterial pathogens. Antimicrob Agent Chemother. 1987. 31:632–634.

21. Gould IM, Milne K. In vitro pharmacodynamic studies of piperacillin/tazobactam with gentamicin and ciprofloxacin. J Antimicrob Chemother. 1997. 39:53–61.
22. Laverdière M, Gallimore B, Restieri C, Poonia K, Chow AW. In vitro synergism of ceftriaxone combined with aminoglicosides against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1994. 19:39–46.

23. den Hollander JG, Horrevorts AM, van Goor ML, Verbrugh HA, Mouton JW. Synergism between tobramycin and ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1997. 41:95–100.

24. Jones RN, Johnson DM, Barrett MS, Erwin ME. Antimicrobial activity of isepamicin (SCH21420, 1-N-HAPA gentamicin B) combinations with cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, imipenem, mezlocillin and piperacillin tested against gentamicin-resistant and susceptible gram-negative bacilli and enterococci. J Chemother. 1991. 3:289–294.

25. Song W, Woo HJ, Kim JS, Lee KM. In vitro activity of beta-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginsa. Int J Antimicrob Agents. 2003. 1:8–12.
26. Burgess DS, Hastings RW. Activity of piperacillin/tazobactam in combination with amikacin, ciprofloxacin and travofloxacin against Pseudomonas aeruginosa by time-kill. Diagn Microbiol Infect Dis. 2000. 38:37–41.

27. Isenberg HD, Alperstein P, France K. In vitro activity of ciprofloxacin, levofloxacin and travafloxacin, alone and in combination with beta-lactams, against clinical isolates of Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia. Diagn Microbiol Infect Dis. 1999. 33:81–86.
28. Neu HC. Synergy and antagonism of combinations with quinolones. Eur J Clin Microbiol Infect Dis. 1991. 10:255–261.

29. Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother. 2002. 50:1045–1049.
30. Drago L, De Vechi E, Nicola L, Colombo A, Guerra A, Gismondo MR. Activity of levofloxacin and ciprofloxacin with cefepime, ceftazidime, imipenem, piperacillin-tazobactam and amikacin against different Pseudomonas aeruginosa phenotypes and Acinetobacter spp. Chemotherapy. 2004. 50:202–210.

31. Mayer I, Nagy E. Investigation of the synergic effects of aminoglycoside-fluoroquinolone and third-generation cephalosporin combinations against clinical isolates of Pseudomonas spp. J Antimicrob Chemother. 1999. 43:651–657.
32. Andes DR, Craig WA. Mandell GL, Bennet JE, Dolin R, editors. Cephalosporins. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 2005. Volume 1:6 th edition. Philadelphia: Churchill Livingstone;294–311.

33. Gilbert DN. Mandell GL, Bennet JE, Dolin R, editors. Aminoglycosides. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 2005. Volume 1:6 th edition. Philadelphia: Churchill Livingstone;328–356.
34. Perry CM, Markham A. Piperasillin/tazobactam an updated review of its use in the treatment of bacterial infections. Drugs. 1999. 57:805–843.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download