Abstract
Purpose
Four pediatric patients with congenital coronary arteriovenous fistula (CAVF) were reported to remind pediatric practitioners and cardiologists of its diagnosis and management.
Materials and Methods
Four pediatric patients with congenital CAVF from June 1999 to November 2007 were included in this retrospective study. Study modalities included reviews of patients' profiles of clinical features, chest radiograph, Doppler echocardiography, cardiac catheterization with angiography, myocardial perfusion scan, and computed tomography.
Results
All 4 patients were symptomatic. The clinical symptoms and signs were feeding problem, continuous murmur, tachycardia, tachypnea, cardiomegaly, and exertional chest pain. Myocardial enzyme was elevated in 1 patient. Echocardiography showed dilatation of the coronary artery in all 4 patients, and traced down its origin in 3 and drainage in 4. The fistulas originated from the right coronary artery in 2 patients and left coronary artery in 2, and were drained into the right ventricle in 2, right atrium in 1, and pulmonary artery in 1. Single left coronary artery was found in 1 patient. The pulmonary-to-systemic blood flow ratios ranged from 1.2 to 2.5. Transcatheter coil occlusion was successfully performed in 4 patients through a coaxial delivery system. The symptoms and signs of congestive heart failure and myocardial ischemia disappeared after the procedure.
Conclusion
Diagnosis of congenital CAVF could be achieved by appreciation of continuous murmur over area unusual for the ductus, and by scrupulous examination of echocardiography as well as angiography of the coronary artery through which coaxial transcatheter coil occlusion could be performed successfully.
Congenital coronary arteriovenous fistula (CAVF) is a rare anomaly through which coronary blood flow is usually shunted into a cardiac chamber, great vessel, or other structures, bypassing the myocardial capillary network to render coronary steal phenomenon and myocardial ischemia, and causing morbidity and mortality.1-5 Surgical intervention had previously been advocated to treat congenital CAVF.6-9 Up to date, transcatheter coil occlusion emerges as the treatment of choice.10-18 In this article, 4 pediatric patients with congenital CAVF presenting congestive heart failure and myocardial ischemia are reported. In addition, the diagnosis, differential diagnosis, and management of congenital CAVF are discussed, and its outcome following transcatheter coil occlusion by means of a coaxial delivery system is reviewed from the English literature.
From June 1999 to November 2007, 4 pediatric patients with congenital CAVF (3 male and 1 female) aged 49 days to 10 years, were treated and included in this retrospective study. Patients with anomalous left coronary artery from pulmonary artery syndrome were excluded. The study modalities included reviews of patients' profiles of clinical manifestations, plain chest radiographs, echocardiography with Doppler, cardiac catheterization with angiography, myocardial perfusion scan, and computed tomography. Informed consent was obtained from all parents. Transcatheter coil occlusion was performed by means of a coaxial delivery system composed of: 1) a 5.2-French Judkins Coronary Catheter (Johnson-Johnson, Miami, FL, USA) or a 5-French Amplatz Right I (Medtronic, Minneapolis, MN, USA) in the outer layer, 2) 2.5-French Target Tracker-18MX Infusion Catheter (Target, Fremont, CA, USA) or 2.4-French Progreat (Terumo, Fujinomiya, Shizuoka, Japan) in the inner layer, and 3) Fibered Platinum Coil followed by Vortx Coil Pusher-16 (Target, Meditech, Watertown, MA, USA) in the central core (Fig. 1). The Amplatz Right I or the Judkins Coronary Catheter may serve as a strong supportive catheter in the outer layer, and the Infusion Catheter of Progreat or the Target Tracker-18MX serve as a flexible "target-tracker catheter" in the inner layer. By this steady coaxial delivery system, the fibered platinum coil could easily be pushed out of the infusion catheter by a pusher wire (Vortx Coil Pusher-16). Two modalities of 0.018-in fibered platinum coils were chosen to occlude the fistulas, including the cone-shaped Vortx-18 and the S-shaped Complex Helical Fibered Platinum Coil-18. Prior to coil occlusion, a coronary balloon catheter of 4.0 mm in diameter and 2.0 cm in length (HAYATE, Terumo, Tokyo, Japan) was inflated for 5 minutes to confirm the absence of myocardial ischemia on electrocardiogram in the catheterization room. Intravenous heparin was given to prevent blood clotting in this coaxial system of delivery beforehand. Aortography and/or selective angiography of the incriminated coronary artery were performed 20 minutes after the procedure to document the final results.
The clinical profiles of 4 pediatric patients with CAVF are summarized in Table 1. All 4 patients were symptomatic, and their ages ranged from 49 days to 10 years. The most common symptoms and signs calling for medical help were feeding problems (n = 3), continuous murmur (n = 3), tachycardia (n = 3), tachypnea (n = 3), cardiomegaly (n = 3), and exertional chest pain with cold sweating (n = 1). Myocardial enzyme was elevated in 1 patient (patient 2). Echocardiography showed dilatation of the coronary artery in 4 patients. The origin and drainage of CAVF could be identified in 3 and 4 patients, respectively. Single left CAVF was documented in patient 2, who presented symptoms and signs of congestive heart failure and myocardial ischemia. Patient 4 was a 10-year-old boy who had suffered from Kawasaki disease at 3 years of age, and was free of symptoms and signs during 7 years of follow up. However, he presented chest pain, cold sweating, and pale face after 100-m dashing and long-distance jogging in the past 3 months. Myocardial perfusion scan or stress/rest 99mtechnetium-methoxyisobutylisonitrile (99mTc-MIBI) single-photon emission computed tomography with a bicycle ergometer, which was performed in order to assess coronary artery stenosis, showed reversible perfusion abnormality in the basal inferoseptal segment, basal inferior segment, mid-inferior segment, and apical inferior segment, indicating myocardial ischemia involving the territory of the right coronary artery. According to angiographic imaging, the fistulas originated from the right coronary artery in 2 patients (patients 1 and 4) and left coronary artery in 2 (patients 2 and 3), and drained into the right ventricle in 2 (patients 1 and 2), right atrium in 1 (patient 3), and the pulmonary artery in 1 (patient 4). The pulmonary-to-systemic blood flow (Qp/Qs) ratios ranged from 1.2 to 2.5. Transcatheter coil occlusion was successfully performed through the coaxial delivery system in all 4 patients. The symptoms and signs of congestive heart failure, continuous murmur, and exertional chest pain disappeared after the procedure in all patients. Myocardial perfusion scan showed regression of reversible perfusion abnormality in patient 4 at 1-year follow up.
Patient 2 was a 15-month-old female who was referred from a pediatric clinic with the chief complaints of heart murmur, postprandial tachycardia, and postprandial dyspnea at the age of 11 months. Vital signs showed increased heart rate of 140 bpm, increased respiratory rate of 32 bpm, and normal blood pressure of 108/68 mmHg. Under sedation by choral hydrate, a faint continuous murmur of grade 2/6 could be appreciated over the right sternal border on chest auscultation. The liver was impalpable. Plain chest radiograph showed cardiothoracic ratio of 55%. There were no pathological Q waves in leads I, aVL, and V5-6 on electrocardiogram. Initially, myocardial enzymes were within normal limits. Doppler echocardiography and cardiac catheterization with angiography tracked down the culprit to be a single left CAVF to the right ventricle. Her parents were hesitant to accept interventional cardiac catheterization at first. Oral nitroglycerin, aspirin, and digitalis were prescribed for the patient at the outpatient clinic of the Division of Pediatric Cardiology. Unfortunately, she suffered from acute bronchiolitis with remarkable tachypnea and tachycardia 4 months later. Thrombocytosis was noted with platelet counts of 1,030,000/mm3. Serum level of the myocardial fraction of creatine kinase (CK-MB) was 15.0 ng/mL (> 6.3 ng/mL) and that of creatine kinase was 186 U/L (> 113 U/L). After speaking to her parents about the high probability of sudden cardiac death provoked by acute coronary occlusion secondary to thrombocytosis or elicited by coronary artery steal phenomenon following any other physical stress or illness and the high success rate and low complication of transcatheter coil occlusion by a coaxial delivery system, they parents finally agreed to interventional cardiac catheterization. We did not prescribe antibiotics for prophylaxis prior to cardiac catheterization. Heparin was administrated intravenously in a dosage of 100 U/kg. At cardiac catheterization, pulmonary artery pressure was 24/14 mmHg (mean, 17), right ventricular pressure 24/5mmHg, and aortic pressure 88/55 mmHg (mean, 74). Qp/Qs ratio was 1.3. Ascending aortography and selective angiography of the left coronary artery (Figs. 2A and B) showed a single left CAVF draining meanderingly to the right ventricle. The maximum diameter of the single left CAVF was 4 mm at the orifice and 2 mm at the termination of the right ventricle. Intravenous heparin was given beforehand to prevent blood clotting within this coaxial system. A 0.014-in PTCA Guide Wire (PT2, Boston Scientific, Miami, FL, USA) was applied to track the single left coronary artery, which was approached at the orifice by a 5-French Amplatz Right I. With the aid of a rail offered by the PTCA Guide Wire, a 2.4-French Infusion Catheter of the "Progreat", which was passed through a 5-French Amplatz Right I, easily tracked this meandering single left CAVF to the distal end (Figs. 2C and D). Transcatheter coaxial coil occlusion was performed after removal of the guide wire using a Vortx Coil Pusher-16 to dislodge 3 sets of 0.018- in fibered platinum coil, 2 of which are of cone-shaped (Vortx) and other S-shaped (Complex Helical) configuration in the looped status after dislodgement. Selective angiography of the left coronary artery 15 minutes after coil occlusion showed complete occlusion of single left CAVF and visualization of the left circumflex coronary artery, implying the presence of insidious coronary artery steal phenomenon caused by this fistulous shunting before (Figs. 2E and F). Continuous murmur disappeared. Doppler echocardiography showed complete occlusion of the fistula. At 3-month follow up, plain chest radiograph, 12-lead surface electrocardiogram and cardiac enzymes were within normal limits. She was free of symptoms and signs of congestive heart failure and myocardial ischemia at 12-month follow up at the outpatient clinic of the Division of Pediatric Cardiology.
It cannot be overemphasized that transcatheter occlusion should be performed with caution, even in case of congenital single left CAVF presenting myocardial ischemia. To the best of our knowledge, this is the first case documenting single left CAVF that was successfully treated by transcatheter coil occlusion through a coaxial delivery system.
Congenital CAVF to a cardiac chamber or a great vessel, which is assumed to appear due to persistence of embryonic intertrabecular spaces and coronary sinusoids, is the most commonly encountered congenital anomaly of the coronary artery that is liable to cause sudden cardiac death.19-21 The natural history and clinical course of congenital CAVF vary significantly among patients affected.4,21,22 It tends to manifest in infants < 2 years of age with congestive heart failure; in young adults with angina, dyspnea on exertion, myocardial ischemia, and myocardial infarction; and in adults > 40 years of age with congestive heart failure, atherosclerosis, and arrhythmias.4
The incidence of congenital CAVF was estimated to be 0.3 - 0.8%.2,23 Congenital CAVF may originate from any of the 3 major coronary arteries, with the right and left anterior descending coronary arteries most commonly involved and the left circumflex coronary artery rarely encountered.5,10-18,24-26 The right coronary artery is incriminated in 55% of cases, left coronary artery in 35%, and both coronary arteries in 5%.5 More than 90% of congenital CAVF drains into the venous circulation (right ventricle, 41%; right atrium, 26%; and pulmonary artery, 17%),19 with extremely rare exceptions into the left ventricle19,27 and pericardium.28
Since congenital CAVF drains most commonly to the right cardiac chambers (right ventricle and right atrium), the single most important clue of the fistula is the location of continuous murmur, which is atypical for the patent ductus arteriosus. Differential diagnosis should include right-side patent ductus arteriosus,29 congenital systemic fistula to the pulmonary vein,30 pulmonary arteriovenous malformation,5 ruptured sinus of Valsalva,31 aortopulmonary window,32 congenital or acquired pulmonary vein stenosis,33 and aortic regurgitation and prolapse of the right coronary cusp associated with supracristal ventricular septal defect.34 Congenital CAVF can be diagnosed by transthoracic35,36 and transesophageal echocardiography23,37,38 with color Doppler flow mapping, and can be defined by laid-back aortography39 of its anatomical features that help facilitate transcatheter coil occlusion.10-18 Some new imaging modalities, including ultrafast computed tomography and magnetic resonance imaging,40-43 may be used as adjunct to coronary angiography to measure abnormal tortuous vessels in 1 section. In pediatric patients with congenital CAVF, however, transthoracic echocardiography and coronary angiography would be sufficient in most circumstances.
Up to date, there has been 1) no consensus in the management strategy for congenital CAVF, 2) no incriminated risk factors in asymptomatic patients with small fistulas, and 3) no cut off value of Qp/Qs bulletining for intervention. Thus, the management remains controversial, and recommendations are based on anecdotal cases or small retrospective series.10-18,44 At one end of the spectrum, the spontaneous closure rate was only 1% in 18 pediatric patients with congenital fistulas, and 16 (asymptomatic in 14 and congestive heart failure in 2) of them were operated.45 At the other end, the closure rate was up to 23% in 31 pediatric patients with clinically silent fistulas to whom conservative management with continued follow-up was recommended.22 Treatment of asymptomatic adult patients is still under debate since most remain free of symptoms for a long time.46,47 However, pediatric patients tend to be symptomatic by virtue of larger fistulas that prompt medical attention and treatment. Generally speaking, patients with larger fistulas or symptoms of congestive heart failure and myocardial ischemia and pediatric patients with high Qp/Qs liable to have symptoms secondary to coronary steal phenomenon should be treated as soon as possible. Somehow, clinical symptoms do not significantly correlate with Qp/Qs.4 Furthermore, some patients with congenital coronary fistulas present symptoms only after provocative test or physical stress or illness, similar to patients 2 and 4 in this report. Sato reported 1 case with a low Qp/Qs of 1.08 presenting symptoms of myocardial ischemia only after the stress/rest 99mTc-MIBI SPECT and 123I-BMIPP scintigraphy, indicating surgical intervention.48 Likewise, patient 4 with a low Qp/Qs ratio of 1.2 in this report developed myocardial ischemia, probably by means of coronary steal phenomenon that reflected on the stress/rest 99mTc-MIBI SPECT with the findings of reversible perfusion abnormality.
Although surgical outcomes of congenital CAVF are good,6-9 catheter-based managements using different devices, including detachable balloon,49 interlocking detachable coils,50 Gianturco coils,10,14-18,27 fibered platinum microcoils,10-13,16,24,25 Amplatzer Duct Occluder,51 Amplatzer Vascular Plug,52 and a combination of multiple devices (Amplatzer muscular VSD Occluder, Amplatzer Duct Occluder, flipper coils, and Gianturco-Grifka vascular occlusion device),53 have been proven to be acceptable alternatives to surgery because of their friendly manipulation, easy feasibility, excellent results of high closure rate, and low morbidity and mortality rates.
Before performance of transcatheter coil occlusion, careful assessment of the size and geometry of coronary fistulas are crucial for selection of coils or other devices to achieve complete occlusion In the present study, we dislodged the fibered platinum microcoils at the distal site of fistulas, which usually have the smallest diameters. From the clinical experience of our patients and those reported in the English literature, the terminal insertion and zenith or nadir of a sharp U-turn or a serpentine S-curve usually possess the narrowest diameters, at which points coils or other devices can be safely dislodged without causing distal migration to the peripheral pulmonary artery. If there is no discernible narrowest site throughout the fistula, larger Gianturco coils (0.035-in. or 0.038-in.) or other occlusive devices should be considered instead.
If we attempt to occlude the fistula at the proximal portion of a coronary artery, balloon occlusion test or angiography is mandatory to see if there is presence of ischemic changes on electrocardiogram or presence of side branch of the target coronary artery in the catheterization room. In order to avoid myocardial dysfunction, ischemia, or even infarction, it is recommended not to occlude the fistula at the proximal portion unless it is geometrically unfeasible for a distal coil occlusion. Balloon occlusion test at the distal portion of the fistula can technically be difficult to obtain the common feature of its meandering, serpentine, or tortuous course. Coronary balloon catheters, with small profiles of 2.0 - 4.0 mm in diameter and 1.5 - 2.0 cm in length can reach more distally and be used for balloon occlusion test or angiography without causing significant drawbacks. If it is difficult to track this small coronary balloon catheter deep to the distal end of the fistula for test or angiography, we recommend a transarterial approach through a coaxial delivery system using a larger 5-Fr Judkins Coronary Catheter or 6-Fr Coronary Infusion Catheter for a strong support at the take-off of the fistula to facilitate guiding a 3-Fr flexible Tracker-18MX Infusion Catheter to advance more distally for precise dislodgement of the fibered platinum microcoils. There are 2 major advantages of a coaxial transarterial approach over traditional transarterial approach (without using a coaxial system) and antegrade (or transvenous) approach. The technique of transarterial approach by a coaxial delivery system involves using smaller sheaths and infusion catheters (3-Fr Tracker-18MX), which can be housed into a larger 5-Fr Judkins Coronary Catheter or 6-Fr Coronary Infusion Catheter to form a steady coaxial system to deliver platinum microcoils more easily and precisely at the distal end of the fistula or at insertion of the right cardiac chambers. A coaxial delivery system offers better stabilization during transcatheter coil occlusion. Clinical application of a detachable coil, umbrella, or balloon, instead of fibered platinum microcoils, requires a larger delivery system that can be difficult to track the larger Judkins Coronary Catheter to the distal end of the coronary fistula. Under such circumstances, a transarterial approach (without using a coaxial system) will inevitably dislodge larger occlusive devices at a more proximal portion, jeopardizing more of the side branches at risk of obstruction. An antegrade or transvenous approach has 2 irksome tasks to overcome. First, a snare catheter may be used to pull a guide wire passed from the aorta through the coronary artery out of the fistula from a venous catheter in the right atrium or right ventricle. Second, it is demanding to track a delivery system deep enough into the coronary fistula en route from the right cardiac chambers to the meandering coronary artery for coil occlusion.25 Only a few cases of antegrade or transvenous approach from the pulmonary artery rather than from the right atrium or right ventricle have been reported.54 Likewise, congenital CAVF to the superior vena cava, azygos vein, and coronary sinus can be approached antegradely or transvenously if indicated for transcatheter coil occlusion.
The clinical profiles of 15 pediatric patients with congenital CAVF who underwent transcatheter coil occlusion by fibered platinum coil through a coaxial delivery system are summarized in Table 2.
In conclusion, diagnosis of congenital CAVF could be achieved by canny appreciation of continuous murmur over area that is unusual for the patent ductus arteriosus, by scrupulous examination of echocardiography, and by selective angiography of the incriminated coronary artery through which transcatheter coil occlusion could be performed simultaneously and successfully by a coaxial delivery system, even in patients presenting congestive heart failure or myocardial ischemia. Single left CAVF did not preclude transcatheter coil occlusion by way of a coaxial delivery system.
Figures and Tables
 | Fig. 1(A) Cross section of the coaxial delivery system, which is composed of a strong "supportive catheter" of either 5-French Amplatz Right I Catheter or 5-French Judkins Coronary Catheter in the outer layer (denoted as Arabic numeral 1), a flexible "target-tracker catheter" of either 2.4-French Progreat or 2.5-French Tracker-18MX Infusion Catheter in the inner layer (denoted as Arabic numeral 2), and (B and C) cone-shaped Vortx-18 or (D) S-shaped Complex Helical Fibered Platinum Coil-18 followed by the Coil Pusher-16 in the central core (denoted as Arabic numeral 3). By means of this coaxial delivery system, two different modalities of Fibered Platinum Coil-18, which were chosen according to the geometry of fistula, could be steadily dislodged at the distal end by the Coil Pusher-16 through the flexible infusion catheter of "Progreat" or "Tracker-18MX" within the meandering congenital CAVF. CAVF, coronary arteriovenous fistula. |
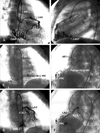 | Fig. 2(A and B) Selective angiography of the left coronary artery, which was entered by a 5-French Amplatz Right I (AR I), showed a single left coronary arteriovenous fistula (CAVF) originating from left anterior descending coronary artery (LAD) draining to the right ventricle. (C and D) With the aid of a rail offered by a 0.014-in PTCA Guide Wire, a 2.4-French Progreat Infusion Catheter (PIC), which was passed through a 5-French Amplatz Right I (AR I) that approached the left coronary artery at the orifice, tracked this meandering single left CAVF to the distal end. Transcatheter coaxial coil occlusion was performed, after removal of the 0.014-in guide wire, using a Vortx Coil Pusher-16 to dislodge 3 sets of 0.018-in Fibered Platinum Coil (FPC). (E and F) Selective angiography of the left coronary artery, 15 minutes. after coil occlusion, showed complete occlusion of the fistula and visualization of the left circumflex coronary artery (LCX), implying presence of insidious coronary artery steal phenomenon caused by this fistulous shunting. |
Table 1
Clinical Profiles of 4 Pediatric Patients with Congenital CAVF Underwent Transcatheter Coil Occlusion through a Coaxial Delivery System

CAVF, coronary arteriovenous fistula; CHF, clinical symptoms and signs of congestive heart failure, including tachycardia, tachypnea (or dyspnea), and cardiomegaly; F, female; FPC, fibered platinum coil; LAD, left anterior descending coronary artery; M, male; MI, myocardial ischemia; PA, pulmonary artery; Qp/Qs, pulmonary-to-systemic flow ratio; RA, right atrium; RCA, right coronary artery; RV, right ventricle.
ACKNOWLEDGMENTS
The author appreciated the assistance of the personnel of the Cardiovascular Laboratory: Jui-Wen Peng, Guo-Jhueng Tu, San-Yi Chen, Jyong-You Lee, and Shu-Lin Chang, and of the Pediatric Special Nurse: Chia-Yi Liu, Tsun Song, and Chiu-Mei Lin.
References
1. Chu E, Cheitlin MD. Diagnostic considerations in patients with suspected coronary artery anomalies. Am Heart J. 1993. 126:1427–1438.
2. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990. 21:28–40.

3. Roberts WC. Major anomalies of coronary arterial origin seen in adulthood. Am Heart J. 1986. 111:941–963.

4. Liberthson RR, Sagar K, Berkoben JP, Weintraub RM, Levine FH. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation. 1979. 59:849–854.

5. Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006. 107:7–10.

6. Kamiya H, Yasuda T, Nagamine H, Sakakibara N, Nishida S, Kawasuji M, et al. Surgical treatment of congenital coronary artery fistulas: 27 years' experience and a review of the literature. J Card Surg. 2002. 17:173–177.

7. Blanche C, Chaux A. Long-term results of surgery for coronary artery fistulas. Int Surg. 1990. 75:238–239.
8. Bogers AJ, Quaegebeur JM, Huysmans HA. Early and late results of surgical treatment of congenital coronary artery fistula. Thorax. 1987. 42:369–373.

9. Urrutia-S CO, Falaschi G, Ott DA, Cooley DA. Surgical management of 56 patients with congenital coronary artery fistulas. Ann Thorac Surg. 1983. 5:300–307.
10. Kung GC, Moore P, McElhinney DB, Teitel DF. Retrograde transcatheter coil embolization of congenital coronary artery fistulas in infants and young children. Pediatr Cardiol. 2003. 24:448–453.

11. Ogoh Y, Akagi T, Abe T, Hashino K, Hayabuchi N, Kato H. Successful embolization of coronary arteriovenous fistula using an interlocking detachable coil. Pediatr Cardiol. 1997. 18:152–155.

12. Beekman RH 3rd, Shim D, Lloyd TR. Embolization therapy in pediatric cardiology. J Interv Cardiol. 1995. 8:543–556.

13. De Wolf D, Terriere M, De Wilde P, Reidy JF. Embolization of a coronary fistula with a controlled delivery platinum coil in a 2-year-old. Pediatr Cardiol. 1994. 15:308–310.

14. Latson LA, Forbes TJ, Cheatham JP. Transcatheter coil embolization of a fistula from the posterior descending coronary artery to the right ventricle in a two-year-old child. Am Heart J. 1992. 124:1624–1626.

15. Perry SB, Rome J, Keane JF, Baim DS, Lock JE. Transcatheter closure of coronary artery fistulas. J Am Coll Cardiol. 1992. 20:205–209.

16. Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ. Transcatheter embolization in the treatment of coronary artery fistulas. J Am Coll Cardiol. 1991. 8:187–192.

17. Moskowitz WB, Newkumet KM, Albrecht GT, Goble MM, Schieken RM. Case of steel versus steal: coil embolization of congenital coronary arteriovenous fistula. Am Heart J. 1991. 121:909–911.

18. Issenberg HJ. Transcatheter coil closure of a congenital coronary arterial fistula. Am Heart J. 1990. 120:1441–1443.

19. Levin DC, Fellows KE, Abrams HL. Hemodynamically significant primary anomalies of the coronary arteries. Angiographic aspects. Circulation. 1978. 58:25–34.

20. Gupta NC, Beauvais J. Physiologic assessment of coronary artery fistula. Clin Nucl Med. 1991. 16:40–42.

21. Lau G. Sudden death arising from a congenital coronary artery fistula. Forensic Sci Int. 1995. 73:125–130.

22. Sherwood MC, Rockenmacher S, Colan SD, Geva T. Prognostic significance of clinically silent coronary artery fistulas. Am J Cardiol. 1999. 83:407–411.

23. Vitarelli A, De Curtis G, Conde Y, Colantonio M, Di Benedetto G, Pecce P, et al. Assessment of congenital coronary artery fistulas by transesophageal color Doppler echocardiography. Am J Med. 2002. 113:127–133.

24. Sreedharan M, Prasad G, Barooah B, Dash PK. Vortex coil embolisation of coronary artery fistula. Int J Cardiol. 2004. 94:323–324.

25. Vance MS. Use of platinum microcoils to embolize vascular abnormalities in children with congenital heart disease. Pediatr Cardiol. 1998. 19:145–149.
26. Olgunturk R, Kula S, Tunaoglu FS. Transcatheter closure of a rare form of coronary arteriovenous fistula (circumflex artery to coronary sinus). Int J Cardiol. 2006. 113:261–263.
27. Papazoglou PD, Mitsibounas D, Nanas JN. Left anterior descending coronary artery-left ventricular fistula presenting as unstable angina and syncope. Int J Cardiol. 2004. 96:121–122.
28. Mutlu H, Serdar-Küçükoğlu M, Ozhan H, Kansýz E, Oztürk S, Uner S. A case of coronary artery fistula draining into the pericardium causing hematoma. Cardiovasc Surg. 2001. 9:201–203.

29. Lee ML, Chaou WT, Wang YM, Fang W, Chiu IS. A new embryonic linkage between chromosome 22q11 deletion and a right ductus from a right aortic arch in a neonate with DiGeorge syndrome. Int J Cardiol. 2001. 79:315–316.

30. Currarino G, Willis K, Miller W. Congenital fistula between an aberrant systemic artery and a pulmonary vein without sequestration. A report of three cases. J Pediatr. 1975. 87:554–557.
31. Lee ML, Wang JK, Wu MH, Chang CI, Lue HC. Echocardiographic and angiographic identification of ruptured sinus of Valsalva and aortic root abscess complicating infective endocarditis in ventricular septal defect: report of two cases. Acta Cardiol Sin. 1995. 11:87–91.
32. Lee ML. Recognition of Berry syndrome in a 4-day-old neonate by echocardiography and transvenous angiocardiography. Int J Cardiol. 1999. 71:93–95.
33. Lee ML, Wang JK, Lue HC. Visualization of pulmonary vein obstruction by pulmonary artery wedge injection and documentation by pressure tracings: report of one case with persistent wheezing following correction of total anomalous pulmonary venous connection. Int J Cardiol. 1995. 49:167–172.
34. Lue HC, Sung TC, Hou SH, Wu MH, Cheng SJ, Chu SH, et al. Ventricular septal defect in Chinese with aortic valve prolapse and aortic regurgitation. Heart Vessels. 1986. 2:111–116.
35. Velvis H, Schmidt KG, Silverman NH, Turley K. Diagnosis of coronary artery fistula by two-dimensional echocardiography, pulsed Doppler ultrasound and color flow imaging. J Am Coll Cardiol. 1989. 14:968–976.

36. Barbosa MM, Katina T, Oliveira HG, Neuenschwander FE, Oliveira EC. Doppler echocardiographic features of coronary artery fistula: report of 8 cases. J Am Soc Echocardiogr. 1999. 12:149–154.
37. Cox ID, Heald SC, Murday AJ. Value of transoesophageal echocardiography in surgical ligation of coronary artery fistulas. Heart. 1996. 76:181–182.

38. Krishnamoorthy KM, Rao S. Transesophageal echocardiography for the diagnosis of coronary arteriovenous fistula. Int J Cardiol. 2004. 96:281–283.

39. Hofbeck M, Wild F, Singer H. Improved visualisation of a coronary artery fistula by the "laid-back" aortogram. Br Heart J. 1993. 70:272–273.

40. Yoshimura N, Hamada S, Takamiya M, Kuribayashi S, Kimura K. Coronary artery anomalies with a shunt: evaluation with electron-beam CT. J Comput Assist Tomogr. 1998. 22:682–686.

41. Ropers D, Moshage W, Daniel WG, Jessl J, Gottwik M, Achenbach S. Visualization of coronary artery anomalies and their anatomic course by contrast-enhanced electron beam tomography and three-dimensional reconstruction. Am J Cardiol. 2001. 87:193–197.

42. Pucillo AL, Schechter AG, Moggio RA, Kay RH, Baum SJ, Herman MV. MR imaging in the definition of coronary artery anomalies. J Comput Assist Tomogr. 1990. 14:171–174.

43. Taoka Y, Nomura M, Harada M, Mitani T, Endo J, Kondo Y, et al. Coronary-pulmonary artery fistulae depicted by multiplanar reconstruction using magnetic resonance imaging. Jpn Circ J. 1998. 62:455–457.

44. Umaña E, Massey CV, Painter JA. Myocardial ischemia secondary to a large coronary-pulmonary fistula-case report. Angiology. 2002. 53:353–357.
45. Farooki ZQ, Nowlen T, Hakimi M, Pinsky WW. Congenital coronary artery fistulae: a review of 18 cases with special emphasis on spontaneous closure. Pediatr Cardiol. 1993. 14:208–213.

46. Said SA, Landman GH. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. Int J Cardiol. 1990. 27:203–210.

47. Sunder KR, Balakrishnan KG, Tharakan JA, Titus T, Pillai VR, Francis B, et al. Coronary artery fistula in children and adults: a review of 25 cases with long-term observations. Int J Cardiol. 1997. 58:47–53.
48. Sato F, Koishizawa T. Stress/Rest 99mTc-MIBI SPECT and 123I-BMIPP scintigraphy for indication of surgery with coronary artery to pulmonary artery fistula. Int Heart J. 2005. 46:355–361.

49. Krabill KA, Hunter DW. Transcatheter closure of congenital coronary arterial fistula with a detachable balloon. Pediatr Cardiol. 1993. 14:176–178.
50. Qureshi SA, Reidy JF, Alwi MB, Lim MK, Wong J, Tay J, et al. Use of interlocking detachable coils in embolization of coronary arterivenous fistulas. Am J Cardiol. 1996. 78:110–113.
51. Thomson L, Webster M, Wilson N. Transcatheter closure of a large coronary artery fistula with the Amplatzer duct occluder. Catheter Cardiovasc Interv. 1999. 48:188–190.
52. Fischer G, Apostolopoulou SC, Rammos S, Kiaffas M, Kramer HH. Transcatheter closure of coronary arterial fistulas using the new Amplatzer vascular plug. Cardiol Young. 2007. 17:283–287.

53. Holzer R, Waller BR 3rd, Kahana M, Hijazi ZM. Percutaneous closure of a giant coronary arteriovenous fistula using multiple devices in a 12-day-old neonate. Catheter Cardiovasc Interv. 2003. 60:291–294.
54. Wax DF, MaGee AG, Nykanen D, Benson LN. Coil embolization of a coronary artery to pulmonary artery fistula from an antegrade approach. Cathet Cardiovasc Diagn. 1997. 42:68–69.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download