Abstract
Materials and Methods
From 1996 to 2004, 94 patients underwent surgery due to papillomas of the breast. Among them, 21 patients underwent 3D fast low angle shot (FLASH) dynamic breast MRI. Eight masses were palpable and 11 of 21 patients had nipple discharge. Two radiologists indifferently analyzed the location, size of the lesions and shape, margin of the masses, multiplicity and ductal relation. The MRI findings were categorized according to breast imaging reporting and data system (BI-RADS) lexicon. The amount and pattern of enhancement and associated findings were also evaluated according to BI-RADS. We then compared the MRI findings with galactography, mammography and breast ultrasonography (US) and examined histopathologic correlation.
Results
On breast MRI, the
lesion size was 0.4-1.59 cm, and 18 patients showed subareolar location. On 4.25 cm (mean 1.54) dynamic enhanced images, imaging findings showed mass (n = 10), intracystic mass (n = 3), focus (n = 5), ductal enhancement (n = 2), and segmental enhancement (n = 1). In cases of the masses, the shapes of the masses were round (n = 4), lobulated (n = 3), and irregular (n = 6), and margins were circumscribed (n = 6), microlobulated (n = 5), and indistinct (n = 2). The enhancement patterns were homogeneous enhancement (n = 7), heterogeneous (n = 3) or rim enhancement (n = 3).
Breast papillomas are one of the most common benign neoplasms in the breast. Breast papillomas include solitary intraductal papilloma, multiple papillomas, papillomatosis, and juvenile papillomatosis (JP),1 and they can be single or multiple, and may or may not be associated with clear or bloodstained nipple discharge.2 They also can be palpable or not. They are difficult to diagnose because of their symptoms and signs can be confused with those of breast cancer. Patients who present with atypical nipple discharge routinely undergo mammography with subsequent galactography. On mammography, papilloma is usually occult, however, may be visible as a solitary dilated duct, a nodule, or a nonspecific cluster of microcalcifications.3-6 On sonography, papillomas may be solid, homogeneously hypoechoic intraductal mass within a dilated duct.3-6 Galactography may reveal an intraluminal filling defect, duct diltation, contrast extravasation, or complete obstruction.3-6 Galactography may reveal and help localize the lesion for biopsy and may also reveal additional lesions.4,5 Although carcinomas are frequently encountered, there are no definitive galactographic criteria for differentiation of benign from malignant lesions. Sometimes solitary or multiple papillomas and papillomatosis are difficult to diagnose by imaging modalities and the role of breast MRI in diagnosis of mammary lesions has increased.2,7,8 Therefore, the purpose of this study was to elucidate the role of 3D dynamic contrast enhanced MRI in the diagnosis and preoperative evaluation of breast papillomas.
The institutional review board approved this study and required neither patients' approval nor patients' informed consent for review of their images and records. From 1996 to 2004, 94 patients were diagnosed with papillomas of the breast at our institute. Among them, 21 patients had taken 3D fast low angle shot (FLASH) dynamic breast MRI and those patients were enrolled in this study. All patients were female, and their age was 27 to 74 years (mean age: 46.3 years). Eleven of 21 patients had nipple discharge, and 2 patients among them showed palpable masses. Eight masses were palpable and 4 patients were asymptomatic. Three patients were diagnosed with breast cancer and took breast MRI for cancer staging, and another 3 patients took MRI for screening of high risk patients. Fifteen patients showed suspicious findings in other imaging modalities. Mammography was available in 17 patients, and 17 patients took breast ultrasonography (US), and 8 underwent galactography.
MRI was performed using a 1.5-T magnet (Magnetom Avanto, Siemens Medical Solution, Erlangen, Germany) with a dedicated bilateral breast surface coil with prone position. The imaging protocol and parameters were as follows: axial T1-weighted image (TR/TE, 500/11) and T2-weighted turbo spin-echo image (5000/100) of both breasts were obtained with 3 mm slice thickness. Next, T1-weighted images were acquired using a 3D FLASH (fast low-angle shot pulse sequence) with a fat-selective inversion for fat suppression through both breasts (TR/TE 3.91/1.42, flip angle 30°). Precontrast images were obtained over a 512 × 317 matrix in the axial plane with a slice thickness of 1.5 mm without gap before administration of the contrast agent. Then, contrast-enhanced dynamic imaging was performed with an injection of 20 mL of gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Wayne, NJ, USA); five sequential contrast-enhanced images were aquired at every 1 min. The precontrast images were then subtracted from the corresponding postcontrast images on a pixel-by-pixel basis with use of the standard software subtraction function available on our console.
MRI examinations were retrospectively reviewed by two radiologists who experienced in the interpretation of breast MRI examinations.
Two radiologists independently analyzed the location, size of lesions, shape, margin of masses, multiplicity and ductal relation. The MRI findings were described according to ACR BI-RADS lexicon and categorized. The amount and pattern of enhancement and associated findings were also evaluated. We compared the MRI findings with galactography, mammography and breast US and examined histopathologic correlation. The US-guided core biopsy was performed using 14G automated guns (Bard-Magnum Biopsy Instruments, Covington, GA, USA). At least three good cores of tissue were obtained for each lesion. BI-RADS category on mammography, sonography, MRI and MRI kinetics were correlated with pathologic findings and nipple discharge. All statistical analysis was performed using SPSS version 11.0 software. All results with a p value of less than 0.05 were considered significant.
On breast MRI, the lesion size was 0.4 to 4.25 cm (mean 1.54 ± 1.59). On dynamic enhanced images, imaging findings included mass (n = 10), intracystic mass (n = 3), focus (n = 5), ductal enhancement (n = 2), and segmental enhancement (n = 1). In case of the masses, the shapes of the masses were round (n = 4), lobulated (n = 3), and irregular (n = 6) and margins were circumscribed (n = 6), microlobulated (n = 5), and indistinct (n = 2). The enhancement patterns of the mass and intracystic mass were homogeneous enhancement (n = 7), heterogeneous (n = 3) or rim enhancement (n = 3). Four cases among mass enhancement had multiple lesions which showed multiple nodular enhancements. Their pathology of those multiple enhanced nodules was papillomas (n = 2), intraductal papilloma with atypical lobular hyperplasia (n = 1) and papillomatsis (n = 1). In terms of non-mass like enhancement, 2 cases showed ductal enhancement and 1 case revealed segmental enhancement. Two cases of ductal enhancement revealed 1 intraductal papilloma and 1 papillomatosis, while segmental enhanced lesion was papillomatosis. Those 2 cases of ductal enhancement indicated negative findings in US. After taken breast MRI, the treatment plans for papillary lesions were changed in 5 patients. The kinetic curve of initial phase showed fast enhancement in all cases, and persistent (n = 15), plateau (n = 5), and wash out pattern (n = 1) in delayed phase. In terms of MR kinetics, the lesions with persistent enhancement pattern (n = 15) revealed intraductal papilloma in 12 cases (80%), 1 papillomas, 1 intraductal papilloma with microcalcifications and 1 papillomatosis with microcalcifications. Otherwise, the lesions with plateau pattern enhancement showed 2 intraductal papilloma, 2 papillomatosis and 1 intraductal papiloma with atypical lobular hyperplasia. Overall, BI-RADS categories of breast MRI were 3 (n = 14), 4a (n = 6), and category 4b (n = 1). Among the 14 lesions diagnosed with BI-RADS 3, their final pathologies were: intraductal papilloma (n = 12, 85.7%), papillomas (n = 1) and papillomatosis with microcalcifications (n=1). On BI-RADS, category 4a lesions were: intraductal papilloma (n = 3), papillomatosis (n = 2) and intraductal papilloma with atypical lobular hyperplasia (n = 1). On pathologic evaluations, the lesion size was 0.3 to 3.83 cm, suggesting mild overestimation of lesion size in MRI study.
Mammography was performed on 17 cases, and the findings were: negative (n = 4), mass (n = 6), asymmetric density (n = 4), and microcalcifications (n = 3). The mass density revealed well-defined high density mass (n = 6), multiple nodular densities (n = 1), and speculated irregular shaped mass (n = 1). The BI-RADS category of mammography was category 1 (n = 4), 3 (n = 4), 4a (n = 1), 4b (n = 2) and 0 (n=6).
On breast ultrasonography (n = 17), the findings were: mass (n = 11), duct dilatation (n = 3), ill-defined heterogeneous echo (n = 1), and negative findings (n = 2). The mass lesion showed solid (n = 8) or intracystic mass (n = 3), and 3 cases among them showed multiple masses. US BI-RADS categories were category 1 (n = 2), 3 (n = 9), 4a (n = 3), and 4b (n = 3).
On galactography, the findings were: single filling defect (n = 4) and multiple filling defects (n = 4).
US-guided core biopsy was performed when possible (n = 7), and another surgical biopsies such as mammo-guided (n = 2) or US-guided (n = 5) localization excision were done. In case of MR showing only lesion with persistent nipple discharge, intraoperative ductography guided (n = 4) excision were performed. According to statistical analysis, the nipple discharge was not correlated with BI-RADS of imaging modalities and breast MRI kinetics (p > .05)
The imaging findings and BI-RADS categories are listed in Table 1.
Breast papillomas are a variety of lesions in the breast that are characterized by a papillary configuration on gross or microscopic examination. These include solitary intraductal papillomas, multiple papillomas, papillomatosis, and juvenile papillomatosis.1 Solitary intraductal papillomas are tumors of major lactiferous ducts, common cause of a serous or serosanguinous nipple discharge. Papilloma is the most common pathologic finding in women with pathologic nipple discharge, accounting for 40% to 70% of cases.9 Multiple intraductal papillomas tend to occur in younger patients, are less often associated with nipple discharge, more frequently peripheral and more often bilateral. Essentially, these lesions appear to be susceptible to the development of carcinoma.10 Carter, et al.11 reported that 2 of 6 patients with multiple papillomas developed cancer, while only 4 of 58 patients with solitary papilloma developed cancer. Another report found that 5 of 51 patients with multiple papillomas developed cancer, which is in marked contrast to 4 of 174 developed cancers with a solitary papilloma. In this series, 32% of simultaneous or subsequent cancers with multiple papillomas were apocrine papillary and cribriform types.12 Papillomatosis is a term used to describe microscopic foci of intraductal hyperplasia, which have papillary architecture without atypia.13
The imaging findings of breast papillomas are very variable, and its pathology is also difficult to diagnose. Breast papillomas are one of the most difficult diagnostic and therapeutic problems, and they could be histologically benign, borderline, or malignant.
On conventional mammography, intraductal papilloma could not be detected, and it has a postitive predictive value of only 25%. The sensitivity is particularly low in young women who have dense breast.14 In our cases, the mammographic findings were negative in 4 cases. We could detect positive mammographic findings in 13 cases among 17 patients: it is because it was diagnostic setting, therefore, the sensitivity was higher than screening setting. In case of microcalcifications, the morphology and distribution revealed suspicious findings. Therefore, so even if other imaging modality showed probable benign findings, further investigations were required.
The galactography is a traditional method for evaluation of the affected duct system in patients with nipple discharge. It is painful and invasive method, and has some complications which include perforation of duct, extravasations of contrast material, and mastitis. These days, therefore, the galactography was replaced by ultrasonography in most of breast centers. Breast papillomas can be demonstrated by filling defects within the dilated duct on galactography. Distortion, narrowing or obstruction of the ducts may indicate malignancy.1 We had 8 cases of galactography in breast papillomas, which showed filling defects or narrowing of duct. However, galactography is a painful procedure and has difficulties in differential diagnosis of benign versus malignancy.
Recently, high resolution ultrasonography is helpful in visualizing intraductal disorders and is becoming a method complementary to traditional radiology techniques.15 However, the sonographic findings of papillomas are variable, and a recent study revealed that sonography is not able to predict malignancy and its positive predictive value is 75%. In our study, the sonographic diagnoses were diverse, and US BI-RADS were 3 to 4b. If patients have nipple discharge or if there was duct dilatation adjacent to the nodules, those findings could possibly have made us to diagnose papillomas. Even more, the ultrasound-guided percutaneous core biopsy and vacuum-assisted large needle biopsy are reliable diagnostic and minimally invasive therapeutic modalities for breast papillomas.
Although MRI is a highly sensitive method for diagnosing breast cancer, its role in the management of papillomas is still controversial.7,8 According to Kramer, et al.,7 combination of breast MRI and galactography could not increase the sensitivity, however, MRI could detect the DCIS which escaped detection with galactography. Daniel, et al., divided the MRI findings of papillomas into 3 groups: 1) small luminal mass papillomas, 2) tumor-like papillomas and 3) MRI-occult papillomas.8 Three groups showed different MRI findings and kinetics. In our cases, there was no case of MRI-occult papilloma. Therefore, the sensitivity of breast MRI of papillomas was 100%. In terms of mass enhancement, the MRI findings were variable. Six masses showed irregular shape enhancement and 13 masses revealed lobulated shape. Therefore, 6 masses among them were MRI BI-RADS category 4a, and 1 was BI-RADS 4b. In our results, there were more papillomatosis or high risk lesions in MRI BI-RADS category 4a than category 3 lesions. If the lesion showed suspicious findings in other imaging modalities, however, the MRI kinetics of the masses showed persistent delayed enhancement, thus making us to consider the possibility of benign mass. There were more papillomatosis or high risk lesions in plateau pattern enhancement than persistent. We had another different pattern of enhancement, such as ductal enhancement or segmental enhancement. Especially, in cases of clinically, mammographically and sonographycally negative breast, breast MRI is helpful for lesion detetion and treatment of nipple discharge. Even there was a mass or asymmetry in mammography or sonography, MRI could find more masses than other image modalities.
The limitation of this study was: the sample size was too small. More samples of papillary lesions of the breasts, including atypism or papillary carcinoma, are needed.
In conclusion, breast MRI could detect papillomas of the breast more than any other image modalities; however its MRI findings were variable. Especially, in terms of multifocal papillomas and papillomatosis, breast MRI plays a key role for evaluation of disease extent.
Figures and Tables
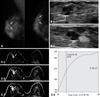 | Fig. 1A 59-year-old woman with palpable mass at 9 : 00 portion of the right breast. She had no symptom of nipple discharge. (A) Right breast mammography showing macolobulated nodule at upper outer area (white arrows). Superficial margin of the mass was obscured. (B) Breast ultrasonography of the mass transverse (1) and longitudinal (2) image revealed isoechoic lobulated mass with some of the margin showing spiculation. Inferior portion of the mass showed dilated duct (arrow in Fig. 1B-1). The sonographic BI-RADS was category 4a, therefore, US-guided core biopsy was recommend. (C) Dynamic contrast breast MRI subtraction 1 minute (1), 3 minutes (2), 5 minutes (3) images. The mass showed lobulated shape, and smooth margin with rim enhancement. Peritumoral delayed enhancement was noted. The kinetics showed persistent enhancement pattern (4). We concluded BI-RADS category 3. The core biopsy and excision were done, and pathologic result was intraductal papilloma. |
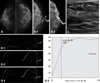 | Fig. 2A 47-year-old woman who had bloody nipple discharge from her left breast for 3 months. (A) Cranial caudal (CC) view of mammography showed heterogeneous dense parenchyma without mass or calcifications. The BI-RADS category was 1, negative finding. (B) Galactography done at left nipple duct revealed intraductal filling defect, and distal portion was not visible (1, white arrows). After injection of more contrast, galactography showed multifocal lobulated filling defects (2, white arrows). (C) Ultrasonography showed focal duct prominency and 0.8 × 0.4 cm sized oval shaped circumscribed nodule with parallel orientation (white arrows), suggesting BI-RADS category 3. (D) Dynamic contrast breast MRI subtraction 1 minute (1), 3 minutes (2), and 5 minutes (3) images demonstrated segmental heterogeneous enhancement. The kinetics showed plateau enhancement pattern (4). We concluded BI-RADS category 4a. Excisional biopsy pathologic result was papillomatosis. |
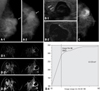 | Fig. 3A 39-year-old woman presented with bloody nipple discharge on her left breast. (A) Left mammography, MLO (1) and CC (2) view showed heterogeneous dense parenchyma pattern and well-circumscribed high density mass lesion at left upper outer portion of breast (white arrow). No evidence of microcalcifications. (B) Breast ultrasonography (a) showed 2.0 × 1.7 cm sized mass lesion at left 2 : 00 portion. The mass was complex cystic mass with internal lobulated hypoechoic solid lesion. Focal ductal extenstion toward the nipple was noted (white arrows). On Doppler image, peripheral increased blood flow was seen. There was no blood flow within the mass (b). (C) On galactography, dilated nipple duct was noted and contrast dye which filled the cystic portion of the mass lesion and solid lesion demonstrated to be lobulated filling defect. (D) Dynamic contrast breast MRI subtraction 1 minute (1), 3 minutes (2), and 5 minutes (3) images. The mass lesion at left 2 : 00 showed peripheral thin rim enhancement. The solid portion of the mass did not reveal enhancement in dynamic images. The enhancement kinetics demonstrated persistent pattern (4), therefore, the BI-RADS category was 3. The pathologic result after excision was intraductal papilloma. |
ACKNOWLEDGEMENTS
This work was supported by Yonsei University Research Fund of 2005 for 6-2005-0089.
This work was supported by Yonsei University College of Medicine Research Fund of 2005 for 6-2005-0101.
References
1. Al Sarakbi W, Worku D, Escobar PF, Mokbel K. Breast papillomas: current management with a focus on a new diagnostic and therapeutic modality. Int Semin Surg Oncol. 2006. 3:1.

2. Francis A, England D, Rowlands D, Bradley S. Breast papilloma: mammogram, ultrasound and MRI appearances. Breast. 2002. 11:394–397.

3. Cardenosa G, Eklund GW. Benign papillary neoplasms of the breast: mammographic findings. Radiology. 1991. 181:751–755.

4. Piccoli CW, Feig SA, Vala MA. Breast imaging case of the day. Benign intraductal papilloma with focal atypical papillomatous hyperplasia. Radiographics. 1998. 18:783–786.

5. Pisano ED, Braeuning MP, Burke E. Diagnosis please. Case 8: solitary intraductal papilloma. Radiology. 1999. 210:795–798.
6. Woods ER, Helvie MA, Ikeda DM, Mandell SH, Chapel KL, Adler DD. Solitary breast papilloma: comparison of mammographic, galactographic, and pathologic findings. AJR Am J Roentgenol. 1992. 159:487–491.

7. Krämer SC, Rieber A, Görich J, Aschoff AJ, Tomczak R, Merkle EM, et al. Diagnosis of papillomas of the breast: value of magnetic resonance mammography in comparison with galactography. Eur Radiol. 2000. 10:1733–1736.

8. Daniel BL, Gardner RW, Birdwell RL, Nowels KW, Johnson D. Magnetic resonance imaging of intraductal papilloma of the breast. Magn Reson Imaging. 2003. 21:887–892.

9. Oyama T, Koerner FC. Noninvasive papillary proliferations. Semin Diagn Pathol. 2004. 21:32–41.
10. Dietz JR, Crowe JP, Grundfest S, Arrigain S, Kim JA. Directed duct excision by using mammary ductoscopy in patients with pathologic nipple discharge. Surgery. 2002. 132:582–587.

11. Carter D. Intraductal papillary tumours of the breast: a study of 78 cases. Cancer. 1977. 39:1689–1692.

12. Haagensen CD, Bodain C, Haagensen DE. Breast carcinoma risk and detection. 1981. Philadelphia: Saunders;146.
13. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985. 312:46–51.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download