Abstract
Purpose
Down-regulation of E-cadherin is a hallmark of the epithelial-to-mesenchymal transition (EMT). EMT progression in cancer cells is associated with the loss of certain epithelial markers and the acquisition of a mesenchymal phenotype, as well as migratory activities. Cyclooxygenase-2 (COX-2) expression is associated with tumor invasion and metastasis in colon cancer. This study investigated the relationship between E-cadherin and COX-2 in colon cancer cells and human colon tumors.
Results
E-cadherin expression was inversely related to the expressions of COX-2 and Snail in colon cancer cells. Ectopic expression of COX-2 or Snail reduced E-cadherin and induced a scattered, flattened phenotype with few intercellular contacts in colon cancer cells. Treatment of cancer cells with phorbol 12-myristate 13-acetate increased the expressions of COX-2 and Snail, decreased 15-hydroxyprostaglandin dehydrogenase expression, and increased the cells' motility. In addition, exposure to prostaglandin E2 increased Snail expression and cell motility, and decreased E-cadherin expression. Membranous E-cadherin expression was lower in adenomas and cancers than in the adjacent, non-neoplastic epithelium. In contrast, the expressions of Snail and COX-2 were higher in cancers than in normal tissues and adenomas. The expressions of COX-2 and Snail increased in areas with abnormal E-cadherin expression. Moreover, COX-2 expression was related to higher tumor stages and was significantly higher in nodal metastatic lesions than primary cancers.
Cyclooxygenase-2 (COX-2), the inducible form of COX, is overexpressed in early and advanced colorectal cancers and is associated with a poor prognosis.1,2 Overexpression of COX-2 or treatment with prostaglandin E2 (PGE2) is related to carcinogenesis via immunosuppression, inhibition of apoptosis, increasing the metastatic potential of epithelial cells, and by promoting angiogenesis.1,2 Conversely, treatment with selective COX-2 inhibitors results in a significant decrease in colorectal adenomas in humans and animals.1,2
Cell-cell adhesion mediated by E-cadherin is required for the maintenance of epithelial tissue architecture in adult organisms.3 Down-modulation of E-cadherin is observed during the later stages of tumorigenesis.4 This down-regulation of E-cadherin is accompanied by a loss of epithelial characteristics and the acquisition of mesenchymal properties, a process often referred to as the epithelial-to-mesenchymal transition (EMT).4 During EMT, carcinoma cells become more motile and invasive by acquiring characteristics similar to embryonic mesenchymal cells, thereby allowing penetration of the stroma surrounding the initial neoplastic focus.5 Control of E-cadherin gene transcription is the primary mechanism accounting for the down-regulation of this protein. The zinc finger factor Snail is a strong repressor of E-cadherin transcription and a well known inducer of EMT. Snail is specifically overexpressed in the tumor cells located at the invasive front. It is responsible for the disruption of E-cadherin mediated cell-cell contacts, invasion, the reduction of tumor proliferative activity, as well as the gain of resistance to apoptosis.6,7 Other E-cadherin repressors that have been implicated in EMT are the Snail family member Slug, the basic helixloop-helix factors E47 and Twist, and the two-handed zinc factors zinc finger E-box-binding homeobox 1 (ZEB1) and and smad-interacting protein 1 (SIP1).5,6
Tsujii and Dubois8 demonstrated that the COX-2 overexpression was linked to inactivation of E-cadherin. A recent study showed that COX-2 and PGE2 expressions in lung cancer cells resulted in a significant reduction of E-cadherin via a ZEB1 and Snail transcriptional factor mediated mechanism, and that inhibition of COX-2 resulted in rescue of E-cadherin expression.9 PGE2 is inactivated by prostaglandin dehydrogenase (PGDH). In human colon cancers, elevated Snail expression correlates well with down-regulation of PGDH.10 Here, we investigated the relationship between E-cadherin and COX-2 in colon cancer cell lines and human colon cancer tissues.
Three colon cancer cell lines (HCT8, HT29, SW620) were obtained from the Korean Cell Line Bank (Seoul, Korea) and grown in RPMI 1640 (Life Technologies, Inc., Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (Life Technologies, Inc.) and gentamicin (10 µg/mL) in a 5% CO2 humidified atmosphere. PGE2 and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma (St. Louis, MO, USA) and dissolved in absolute ethanol and dimethyl sulfoxide, respectively.
HCT8 and SW620 cells (2×105 in 2 mL of RPMI 1640 without gentamicin) were plated in 6-well plates. Twenty-four hours later the cells were transfected with vector alone or with 4 µg of pcDNA3.1-Snail (a gift of Dr. Yook, College of Medicine, Yonsei University, Seoul, Korea) or pCB7-rat COX-2 (a gift of Dr DuBois, Vanderbilt University, TN, USA) using Lipofectamine 2000 (Life Technologies, Inc.) according to the manufacturer's instructions. Briefly, 4 µg of DNA was mixed with 250 µL of Opti-MEM I (Life Technologies, Inc.). The diluted DNA was combined with 10 µL of Lipofectamine 2000 diluted by 250 µL of Opti-MEM I (Life Technologies, Inc.) and incubated for 20 minutes at room temperature. This mixture was added to cells that had attained 90% confluence in 6-well plates. After 48 hours, the cells were harvested for Western blot analysis.
Cells were washed with cold phosphate buffer solution (PBS) and suspended in lysis buffer (1×PBS, 1% Triton X-100) supplemented with complete mini protease inhibitor mixture tablets (Boehringer Mannheim, Mannheim, Germany) on ice for 30 minutes. After removing cell debris by centrifugation, cell lysate protein concentrations were determined using BCA protein assay reagent (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard. Forty µg of protein was separated by 12% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris buffered saline (TBS) for 1 hour at room temperature and probed with antibodies overnight at 4℃. The antibodies used were anti-COX-2 (1 : 1000; Cayman Chemical, Ann Arbor, MI, USA), anti-15-PGDH (1 : 500; Cayman Chemical), anti-E-cadherin (1 : 1000, Transduction Laboratories, Lexington, KY, USA), anti-Snail (1 : 1000, Abcam plc. Cambridge, UK) and anti-glyceraldehydes 3-phosphate dehydrogenase (GAPDH) (1: 200, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After washing with triethanolamine-buffered saline (TBS)-0.05% Tween 20, the blots were treated with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody (1 : 3000; Zymed Laboratories Inc., San Francisco, CA, USA) or a anti-mouse IgG antibody (1 : 3000; Zymed Laboratories Inc.) for 1 hour at room temperature. Detection was performed by enhanced chemiluminescence (Pierce) and autoradiography.
Total RNA was extracted and purified from HT29 cells using a GeneElute Mammalian Total RNA kit (Sigma) according to the manufacturer's instructions. RNA was quantified by measuring absorbance at 260 nm. Two µg of the total RNA from each sample was reverse transcribed into complementary DNA (cDNA) using Superscript II reverse transcriptase (Life Technologies, Inc.) and random hexamer primers (Takara, Shiga, Japan). The Polymerase chain reaction (PCR) primers used were as follows (D) : ZEB1, 5'-TTCAGCATCACCAGGCAGTC-3' (sense), 5'-GAGTGGAGGAGGCTGAGTAG-3' (antisense); Snail, 5'-TTCCAGCAGCCCTACGACCAG-3' (sense), 5'-GCCTTTCCCACTGTCCTCATC-3' (antisense); GAPDH, 5'-GAACGGGAAGCTCACTGGCATGGC-3' (sense), 5'-AATGGTAATGTCAGCTGTATTACT-3'(antisense).11 The PCR conditions used were: 94℃ for 5 minutes, 30 cycles of amplification (94℃ for 45 seconds, 57℃ for 45 seconds, 72℃ for 45 seconds), and an additional extension step of 72℃ for 7 minutes. PCR products were electrophoresed in a 1% agarose gel containing ethidium bromide and then photographed under ultraviolet light.
Confluent cell monolayers were wounded by manually scraping the cells with a pipette tip. Debris was removed from the culture by washing it twice with PBS. The cells were then incubated with serum-free medium including either PGE2 (5 µg/mL) or PMA (50 ng/mL) and allowed to migrate into the wounded area for up to 24 hours at 37℃. Images were immediately acquired at 4 hours and 24 hours after wounding. This test was performed in triplicate.
We used 66 paraffin-embedded primary colon cancers (age range 31-92 years; 30 male and 36 female), 25 metastatic lymph nodes, and 10 adenomas (age range 35-60 years; 5 male and 5 female) obtained from the Department of Pathology at Dongguk University Kyongju Hospital. Formalin fixed paraffin embedded tissue sections of 4 µm thickness were made and spread on poly-L-lysine coated slides. The sections were deparaffinized and hydrated in a graded series of alcohol. Antigen retrieval was routinely performed by immersing the sections in 0.01 M citrate buffer (pH 6.0) in a pressure cooker by autoclaving for 15 minutes. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 minutes and then incubated with a primary antibody for 2 hours at room temperature. The primary antibodies used were: anti-COX-2 (Cayman Chemical, dilution 1 : 500), -E-cadherin (1 : 1000, Transduction Laboratories), and anti-Snail (1 : 500, Abcam plc). Staining was done with an EnVision kit labeled with peroxidase (DAKO, Santa Barbara, CA, USA) and developed with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed Laboratories, South San Franciso, CA, USA) as a chromogen. Sections were counterstained for 3 minutes with Mayer's hematoxylin and then mounted. As a negative control, rabbit or mouse IgG isotype was used instead of primary antibody.
We assessed immunohistochemical staining in only epithelial and tumor cells. Immunohistochemical staining for Snail was defined as a detectable immunoreaction in the nucleus. Immunoreactivity of COX-2 and Snail was evaluated by assessing the proportion of positive cells and graded as absence (no staining), mild (1-30%), moderate (31-60%), or severe (> 60%). Four immunohistochemical staining grades were divided into a positive (moderate and severe) and a negative one (absence and mild) for statistical analysis. Membranous E-cadherin expression was graded according to the proportion of positive cells and classified into a positive group (> 90%) and an abnormal group (0-90%).
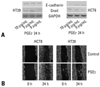
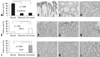
We performed immunoblot analyses for E-cadherin, Snail, and COX-2 in colon cancer cell lines. As shown in Fig. 1A, E-cadherin expressions were higher in HCT8 and HT29 than in SW620, which had strong expressions of Snail and COX-2. In order to verify these findings, HCT8 and SW620 were transfected with cDNA for either COX-2 or Snail, and then harvested after 48 hours. As shown in Fig. 1B and C, ectopic expression of COX-2 or Snail reduced E-cadherin in HCT8 and SW620, and induced a scattered, flattened phenotype with few intercellular contacts in HCT8. As shown in Fig. 2A, PMA treatment (50 ng/mL) increased the expressions of COX-2 and Snail, and decreased the expressions of E-cadherin and PGDH in HCT8. Similar findings were observed in HT29 except for COX-2. The expression of COX-2 was not altered under PMA exposure. Wound healing assays were performed to examine the effects of PMA on cancer cells' motility. As shown in Fig. 2B, PMA treatment increased cell motility in HCT8 and HT29. These results suggest that there is a close association between PG metabolism and cell migration. Thus, we examined the effect of PGE2 treatment on cell motility. As shown in Fig 3, PGE2 exposure for 24 hours increased Snail expression and decreased E-cadherin in HCT8. However, PGE2 exposure did not alter the expression of E-cadherin and slightly increased Snail expression in HT 29. Cell motility was increased in HCT8 and HT29 cells treated with PGE2 (5 µg/mL). As the alteration of Snail expression was weak in HT29 treated with PMA, we examined the messenger RNA (mRNA) levels of Snail and ZEB1 in HT29. As shown in Fig. 4, mRNA levels of snail and ZEB1 were increased in cells treated with either PMA or PGE2.
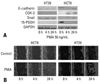
Immunohistochemical results for E-cadherin, COX-2 and Snail in non-neoplastic epithelium, adenomas, and colon cancers are summarized in Table 1. An immunopositive result for membranous E-cadherin was 100% in adjacent non-neoplastic epithelium, 20% in adenomas, and 23% in cancers. As shown in Fig. 5A, membranous E-cadherin expressions were significantly more abnormal in cancers and adenomas than in non-neoplastic epithelium (p < 0.001). Immunoreactivity for Snail was mild in 100% of non-neoplastic epithelium and adenomas, and in cancers it was mild in 36%, moderate in 45% and severe in 18%. Immunoreactivity for COX-2 in non-neoplastic epithelium was absent in 84% and moderate in 16%, and in adenomas, it was absent in 60% and mild in 40%, and in cancers, it was absent in 18%, mild in 39%, and moderated in 42%. As shown in Fig. 5E and 5I, the expressions of Snail and COX-2 were significantly higher in cancers than in adenomas and non-neoplastic epithelium (p < 0.05). The immunopositive results for COX-2 were 14% in stages 0 and I and 50% in stages II-IV. COX-2 expression was significantly related to a higher tumor stage (p = 0.016). There were no significant differences between tumor stage and the immunopositive results for Snail and membranous E-cadherin. We examined the immunoreacitivities of membranous E-cadherin, COX-2, and Snail in primary cancers and metastatic lesions of lymph nodes. COX-2 was immunopositive in 42% of primary cancers and 71% of nodal tumors. As shown in Fig. 6, COX-2 expression was significantly higher in nodal metastatic lesions than primary tumors (p = 0.021). There were no significant differences in the expressions of membranous E-cadherin and Snail between primary cancers and nodal metastatic lesions. As shown in Fig. 7, membranous E-cadherin was predominantly expressed at a tumor center, where COX-2 and Snail were weakly expressed. In contrast, COX-2 and Snail were frequently expressed at the invasive front area, where membranous E-cadherin expression was abnormal.
EMT occurs via 2 distinct biological events: the breaking of cell-cell adhesions and a consequent increase in cell motility.12 A defining characteristic of EMT is the loss of E-cadherin.4 The loss of E-cadherin occurring during carcinoma progression can take place through gene mutations, hypermethylation of its promoter, and transcriptional repression.13 E-cadherin repressors that have been implicated in EMT are the Snail family, the basic helix-loop-helix factors E47 and Twist, and the two-handed zinc factors ZEBl and SIPl.6
In this study, we observed that E-cadherin expression was inversely related to the expressions of COX-2 and Snail in colon cancer cells. Ectopic expression of COX-2 or Snail reduced E-cadherin and induced a scattered, flattened phenotype with few intercellular contacts in colon cancer cells. PMA treatment for cancer cells increased the cells' motility associated with increased expressions of COX-2 and Snail, and a reduction of PGDH expression. PGE2 exposure also increased Snail expression and cell motility, and decreased E-cadherin expression. Tsujii and Dubois8 have demonstrated that COX-2 overexpression in rat intestinal epithelial cells leads to increased adhesion to extracellular matrix related to loss of E-cadherin expression. E-cadherin was upregulated and localized at intercellular junctions and the cytoplasmic membrane in etodolac-stimulated Caco-2 colon cancer cells.14 Recent studies of non-small cell lung cancer and colon cancer have shown that augmentation of PGE2 by COX-2 activation or PGDH inactivation repressed E-cadherin expression through the induction of Snail and ZEB1.9,10 In colon cancer cells, the RhoA/Rho kinase pathway stimulated by COX-2 reduced the levels of E-cadherin and α-catenin leading to a disruption of adherens junction formation and increased cell motility.15 These findings suggest that COX-2 is related to the invasion and metastasis of cancer cells through EMT.
We performed immunohistochemistry for E-cadherin, COX-2, and Snail in primary and metastatic nodal tumors in order to examine their biologic roles. A reduction or loss of E-cadherin expression together with increased COX-2 expression has been reported in colorectal cancers.16 We observed similar results in colon cancers. Previous studies also reported that high expression of COX-2 correlated with a more advanced Dukes' stage.17-19 Our study showed that COX-2 immunopositivity was significantly related to higher tumor stages, and was significantly higher in nodal metastatic lesions than primary cancers. Moreover, in primary cancer lesions, COX-2 and Snail were predominantly expressed at the invasive front, where E-cadherin expression was abnormal. Overall, the reduction or loss of E-cadherin expression, together with increased COX-2 expression, plays an important role for the progression of colon cancers. Based on our in vitro findings in colon cancer cells, we sought to determine if the reciprocal expressions of E-cadherin and COX-2 were evident in cancer cells in situ. As shown in Fig. 7, expressions of COX-2 and Snail increased in the areas with abnormal E-cadherin expression. This finding was also reported in non-small cell lung cancers.9
In conclusion, this study suggests that COX-2 may have a role in the progression of colon cancer through EMT. Thus, blocking PGE2 production or activity may contribute to the treatment and prevention of colon cancer.
Figures and Tables
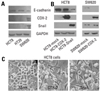 | Fig. 1Western blot analyses of E-cadherin, COX-2 and Snail in colon cancer cells, and morphology of HCT8 transfected with cDNA for COX-2 or Snail. (A and B) Endogenous expressions of E-cadherin, COX-2, and Snail in colon cancer cells, and ectopic expressions of COX-2 and Snail in HCT8 and SW620. Forty µg of protein was separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents GAPDH, which was used as a loading control. (C) Ectopic expression of COX-2 or Snail induced a scattered, flattened phenotype with few intercellular contacts in HCT8. COX-2, cyclooxygenase-2; SDS, sodium dodecyl sulfate; cDNA, complementary DNA; GAPDH, glyceraldehydes 3-phosphate dehydrogenase. |
 | Fig. 2Effects of PMA on the expressions of E-cadherin, COX-2, Snail and 15-PGDH, and cell mobility in HCT8 and HT29. (A) Western blot analyses for E-cadherin, COX-2, Snail and 15-PGDH in the presence of PMA (50 ng/mL) at the indicated times. Forty µg of protein was separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents GAPDH, which was used as a loading control. (B) Cells were incubated with serum-free medium including PMA (50 ng/mL) and allowed to migrate into the wound area for up to 24 hours at 37℃. Images were acquired immediately, and at 4 hours and 24 hours after wounding. PMA (50 ng/mL) treatment increased cell mobility in HCT8 and HT29. COX-2, cyclooxygenase-2; GAPDH, glyceraldehydes 3-phosphate dehydrogenase; PMA, phorbol 12-myristate 13-acetate; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase. |
 | Fig. 3Effects of PGE2 on the expressions of E-cadherin and Snail, and cell mobility in HCT8 and HT29. (A) Western blot analyses for E-cadherin and Snail in the presence of PGE2 at the indicated doses for 24 hours. Forty µg of protein was separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The bottom represents GAPDH, which was used as a loading control. (B) Cells were incubated with serum-free medium including with PGE2 (5 µg/mL) and allowed to migrate into the wound area for up to 24 hours at 37℃. Images were acquired immediately, and at 24 hours after wounding. PGE2 (5 µg/mL) treatment increased cell mobility in HCT8 and HT29. GAPDH, glyceraldehydes 3-phosphate dehydrogenase; PGE2, prostaglandin E2. SDS, sodium dodecyl sulfate. |
 | Fig. 4mRNA expressions for snail and ZEB1 by RT-PCR in HT29 treated with PMA (50 ng/mL) or PGE2 (5 µg/mL for 24 hours. mRNA, messenger RNA; RT-PCR, reverse transcription-polymerase chain reaction; PMA, phorbol 12-myristate 13-acetate; PGE2, prostaglandin E2. |
 | Fig. 5Immunohistochemical staining for E-cadherin (B, C, D), COX-2 (F, G, H) and Snail (J, K, M) in non-neoplastic adjacent mucosa (B, F, J), adenomas (C, G, K), and cancers (D, H, M). (A) Membranous E-cadherin expressions were significantly abnormal in cancers and adenomas than in non-neoplastic epithelium (p < 0.001). (E, I) In contrast, Snail and COX-2 expressions were significantly higher in cancers than in adenomas and non-neoplastic epithelium (p < 0.05, p < 0.001). COX-2, cyclooxygenase-2. |
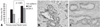 | Fig. 6Immunohistochemical staining for COX-2 in nodal metastatic (B) and primary cancers (C). (A) COX-2 expression was significantly higher in nodal metastatic lesions than primary cancers (p = 0.021). COX-2, cyclooxygenase-2. |
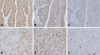 | Fig. 7Topographic distributions of E-cadherin (A, D), Snail (B, E), and COX-2 (C, F) in colon cancers by immunohistochemistry. (A, B and C) Expressions of COX-2 and Snail were decreased in the areas with positive expression of E-cadherin. (D, E, F) In contrast, expressions of COX-2 and Snail increased in the areas with abnormal E-cadherin expression (D). COX-2, cyclooxygenase-2. |
References
1. Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005. 23:2840–2855.

3. Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays. 1999. 21:211–220.

4. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002. 2:442–454.

5. Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007. 39:305–318.

6. Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004. 48:365–375.

7. Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005. 132:3151–3161.

8. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.

9. Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E (2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006. 66:5338–5345.

10. Mann JR, Backlund MG, Buchanan FG, Daikoku T, Holla VR, Rosenberg DW, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006. 66:6649–6656.

11. Barberà MJ, Puig I, Domínguez D, Julien-Grille S, Guaita-Esteruelas S, Peiró S, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004. 23:7345–7354.

12. Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005. 65:10938–10945.

13. Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancer. Am J Pathol. 1998. 153:333–339.

14. Noda M, Tatsumi Y, Tomizawa M, Takama T, Mitsufuji S, Sugihara H, et al. Effects of etodolac, a selective cyclooxygenase-2 inhibitor, on the expression of E-cadherin-catenin complexes in gastrointestinal cell lines. J Gastroenterol. 2002. 37:896–904.

15. Chang YW, Marlin JW, Chance TW, Jakobi R. RhoA mediates cyclooxygenase-2 signaling to disrupt the formation of adherens junctions and increase cell motility. Cancer Res. 2006. 66:11700–11708.

16. Jungck M, Grünhage F, Spengler U, Dernac A, Mathiak M, Caspari R, et al. E-cadherin expression is homogeneously reduced in adenoma from patients with familial adenomatous polyposis: an immunohistochemical study of E-cadherin, beta-catenin and cyclooxygenase-2 expression. Int J Colorectal Dis. 2004. 19:438–445.
17. Ohta T, Takahashi M, Ochiai A. Increased protein expression of both inducible nitric oxide synthase and cyclooxygenase-2 in human colon cancers. Cancer Lett. 2006. 239:246–253.

18. Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002. 97:1037–1041.
19. Nosho K, Yoshida M, Yamamoto H, Taniguchi H, Adachi Y, Mikami M, et al. Association of Ets-related transcriptional factor E1AF expression with overexpression of matrix metalloproteinases, COX-2 and iNOS in the early stage of colorectal carcinogenesis. Carcinogenesis. 2005. 26:892–899.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download